Nanomagnetic Chitosan Composites for Heavy Metal Removal: A Comparative Review of Efficiency, Mechanisms, and Applications
This article provides a systematic analysis of nanomagnetic chitosan composites, a leading adsorbent class for heavy metal remediation in wastewater.
Nanomagnetic Chitosan Composites for Heavy Metal Removal: A Comparative Review of Efficiency, Mechanisms, and Applications
Abstract
This article provides a systematic analysis of nanomagnetic chitosan composites, a leading adsorbent class for heavy metal remediation in wastewater. Tailored for researchers and scientists, it explores the foundational principles, synthesis methodologies, and adsorption mechanisms underpinning their high performance. The content critically evaluates and compares the removal efficiencies of various composite formulations for priority metals like Pb(II), Cr(VI), Cu(II), and Cd(II). Furthermore, it addresses key optimization challenges, regeneration potential, and validation strategies, offering a comprehensive resource for developing advanced, application-ready water purification technologies.
The Science Behind the Sorbent: Unpacking Nanomagnetic Chitosan Composites
Heavy Metals: A Persistent Threat to Ecosystems and Health
Heavy metals are naturally occurring elements in the Earth's crust, but human activities have significantly increased their environmental concentrations, making them persistent and hazardous pollutants [1]. Unlike organic pollutants, heavy metals are non-biodegradable, enabling them to accumulate in the environment and biomagnify through the food chain, ultimately posing severe risks to human health and ecosystems [2] [3] [4].
The toxicity of heavy metals stems from their ability to interfere with essential biological functions. Primary mechanisms include the generation of reactive oxygen species (ROS), leading to oxidative stress that can cause DNA damage, protein modification, and lipid peroxidation [2]. Furthermore, heavy metals can deplete antioxidants like glutathione and bind to sulfhydryl groups of proteins, disrupting enzyme activity [2] [1]. Some metals, such as lead and cadmium, exert toxicity by displacing essential metals like calcium and zinc from their native binding sites in proteins, causing cellular dysfunction [1]. Chronic exposure to heavy metals is linked to neurological disorders, organ damage, and an increased risk of cancer, highlighting the critical need for their effective removal from water and soil [3] [1].
Nanomagnetic Chitosan Composites: A Synergistic Solution
A particularly advanced and promising approach for heavy metal removal involves the use of nanomagnetic chitosan composites. These materials synergistically combine the advantageous properties of two components:
- Chitosan: A natural, biodegradable, and non-toxic biopolymer derived from chitin. Its molecular structure features abundant amino (
-NH₂) and hydroxyl (-OH) functional groups, which act as effective coordination and adsorption sites for heavy metal ions [5] [6]. - Magnetic Nanoparticles (e.g., Magnetite/ Maghemite): These components provide the composite with magnetic susceptibility, allowing for the easy and rapid separation of the spent adsorbent from treated water using an external magnetic field, thereby overcoming challenges associated with filtration or centrifugation [5] [6] [7].
The composite's versatility allows for modifications that enhance its stability, surface area, and adsorption capacity for various heavy metal ions, making it a subject of intense research within the scientific community [4].
Comparative Removal Efficiency of Nanomagnetic Chitosan Composites
The performance of adsorbents is typically evaluated based on their adsorption capacity, measured in milligrams of metal adsorbed per gram of adsorbent (mg/g). The table below summarizes the reported maximum uptake capacities of different magnetic chitosan-based composites for various heavy metals, providing a direct comparison of their efficiency.
Table 1: Adsorption Capacity of Magnetic Chitosan Composites for Heavy Metals
| Composite Name | Target Heavy Metal | Maximum Adsorption Capacity (mg/g) | Key Functional Groups | Source |
|---|---|---|---|---|
| Magnetic Chitosan Composite (MCC) | Pb(II) | 220.9 mg/g | Amino, Hydroxyl | [5] [6] |
| Magnetic Chitosan Composite (MCC) | Cu(II) | 216.8 mg/g | Amino, Hydroxyl | [5] [6] |
| Magnetic Chitosan Composite (MCC) | Ni(II) | 108.9 mg/g | Amino, Hydroxyl | [5] [6] |
| Chitosan-coated MNPs (CMNP) | Pb(II) | ~100% removal efficacy | Amino, Hydroxyl | [7] |
The data demonstrates that magnetic chitosan composites exhibit high affinity for prevalent toxic metals like lead, copper, and nickel. The variation in capacity highlights the material's selectivity, with a particularly high efficiency for lead removal.
Experimental Protocols for Composite Synthesis and Evaluation
To ensure the reproducibility of research in this field, standardized experimental protocols are essential. The following sections detail common methodologies for synthesizing magnetic chitosan composites and evaluating their adsorption performance.
Synthesis of Chitosan-Coated Magnetic Nanoparticles
One efficient method for synthesis is one-step reverse microemulsion precipitation [7].
- Objective: To synthesize chitosan-coated magnetic nanoparticles (CMNP) in a single step with controlled size and high magnetization.
- Materials: Ferric chloride (FeCl₃), ferrous sulfate (FeSO₄), chitosan (low molecular weight), ammonia solution (precipitating agent), toluene, surfactant (e.g., sodium dodecyl sulfate), and deionized water.
- Procedure:
- Prepare a reverse microemulsion by mixing an aqueous phase (containing dissolved chitosan and Fe salts) with a surfactant-toluene mixture. The system is maintained at an elevated temperature (70-80°C) under a nitrogen atmosphere.
- Add an ammonia solution to the microemulsion under vigorous stirring to co-precipitate the magnetic nanoparticles within the chitosan matrix.
- The black precipitate (CMNP) is collected using a magnet, washed repeatedly with deionized water and ethanol to remove impurities, and then dried.
- Characterization: The resulting nanoparticles are characterized using X-ray Diffraction (XRD) to confirm crystal structure and crystallite size (often around 4.5 nm), Fourier-Transform Infrared Spectroscopy (FTIR) to verify chitosan coating, and Vibrating Sample Magnetometry (VSM) to measure saturation magnetization (e.g., 49-53 emu/g) [7].
Batch Adsorption Experiment Protocol
Batch adsorption studies are the standard for evaluating metal removal efficiency [5] [6].
- Objective: To determine the adsorption capacity and kinetics of a composite for specific heavy metal ions under controlled conditions.
- Materials: Composite adsorbent, heavy metal salt solution (e.g., Pb(NO₃)₂, CuCl₂), buffer solutions for pH adjustment, orbital shaker, and Atomic Absorption Spectrophotometer (AAS) or Inductively Coupled Plasma (ICP) instrument for metal concentration analysis.
- Procedure:
- A known mass of the composite (e.g., 10-50 mg) is added to a series of flasks containing a fixed volume (e.g., 100 mL) of metal ion solution at a known initial concentration.
- The pH of the solution is adjusted to the optimal value (often between pH 5-7) using dilute NaOH or HNO₃.
- The flasks are agitated in a shaker at a constant speed and temperature until adsorption equilibrium is reached (typically 120-180 minutes). Samples are taken at regular intervals.
- The composite is separated magnetically, and the residual metal concentration in the supernatant is measured using AAS/ICP.
- Data Analysis:
- Adsorption Capacity (qₑ): Calculated as ( qe = \frac{(C0 - Ce)V}{m} ), where ( C0 ) and ( C_e ) are initial and equilibrium concentrations (mg/L), ( V ) is solution volume (L), and ( m ) is adsorbent mass (g).
- Kinetics: Data is fitted to models like the pseudo-first-order or pseudo-second-order model to understand the adsorption rate.
- Isotherms: Data is fitted to models like Langmuir or Freundlich to describe the adsorption equilibrium.
The Scientist's Toolkit: Essential Research Reagents and Materials
Research into nanomagnetic chitosan composites requires a range of specific reagents and analytical tools. The following table lists key materials essential for experiments in this field.
Table 2: Essential Research Reagents and Materials for Composite Development
| Reagent/Material | Function/Application | Specific Example |
|---|---|---|
| Chitosan (Low MW) | Primary biopolymer matrix providing adsorption sites; its low molecular weight improves processability. | Functional groups (-NH₂, -OH) for metal ion coordination [7]. |
| Ferric Chloride (FeCl₃) | Iron precursor for the synthesis of magnetic nanoparticles (Magnetite/Maghemite). | Fe³⁺ source in co-precipitation reactions [8] [7]. |
| Ferrous Sulfate (FeSO₄) | Iron precursor for the synthesis of magnetic nanoparticles (Magnetite/Maghemite). | Fe²⁺ source in co-precipitation reactions [8] [7]. |
| Ammonia Solution (NH₄OH) | Precipitating agent to form iron oxide nanoparticles from Fe salts. | Creates alkaline conditions for Fe₃O₄ co-precipitation [7]. |
| Lead Nitrate (Pb(NO₃)₂) | Model heavy metal salt for testing adsorption performance and efficiency. | Preparation of stock solutions for batch experiments [7]. |
| Atomic Absorption Spectrophotometer (AAS) | Analytical instrument for quantifying heavy metal ion concentration in solution. | Measuring residual Pb²⁺ concentration after adsorption [6]. |
| Vibrating Sample Magnetometer (VSM) | Characterizes the magnetic properties of the synthesized composite. | Confirming superparamagnetism and saturation magnetization [6] [7]. |
Mechanisms of Metal Adsorption and Composite Regeneration
The high efficiency of nanomagnetic chitosan composites is attributed to their multifaceted adsorption mechanisms. The primary interactions between the composite and heavy metal ions are illustrated below.
A critical advantage of these advanced composites is their potential for regeneration and reuse. After adsorption, the metal-loaded composite can be treated with a mild acidic solution (e.g., 0.1M HNO₃ or EDTA), which desorbs the metal ions from the functional groups. The regenerated composite can then be washed, neutralized, and used in multiple treatment cycles, enhancing its cost-effectiveness and reducing chemical waste [3]. Studies have shown that chitosan microspheres can be regenerated for up to five cycles with consistent performance, underscoring their practical applicability [3].
The pervasive threat of heavy metal contamination in water resources demands the development of efficient, cost-effective, and environmentally friendly remediation technologies. Among various treatment methods, biosorption has emerged as a promising alternative, with chitosan standing out due to its exceptional metal-binding capacities [9]. This natural biopolymer, derived from chitin, offers the dual advantage of sustainability and high performance [10]. The innate affinity of chitosan for metal ions is primarily governed by its unique chemical structure, rich in amino and hydroxyl functional groups [11]. Recent research has focused on enhancing chitosan's properties through the development of nanomagnetic chitosan composites, which combine high sorption efficiency with facile magnetic separation [9] [12]. This guide provides a comparative analysis of chitosan's role as a biosorbent, examining its sources, structural properties, and the performance of its advanced composites against other common adsorbents, supported by experimental data and protocols.
Source and Production of Chitosan
Chitosan is a linear polysaccharide produced by the alkaline deacetylation of chitin [10] [13]. Chitin, the second most abundant natural polymer after cellulose, is a primary component of the exoskeletons of crustaceans such as shrimp, crabs, and prawns, as well as the cell walls of fungi [10] [13].
The industrial production of chitosan from crustacean shells involves four key steps [10]:
- Demineralization (DM): Removal of inorganic materials (like calcium carbonate) using dilute acid.
- Deproteinization (DP): Removal of proteins using alkaline solutions.
- Decolorization (DC): Removal of pigments.
- Deacetylation (DA): Treatment with concentrated sodium hydroxide to remove acetyl groups from chitin, converting it into chitosan.
The resulting product is a copolymer of N-acetyl-D-glucosamine and D-glucosamine units [10]. The degree of deacetylation (DD), which typically ranges from 60% to 95%, significantly influences chitosan's properties, including its solubility and number of available free amino groups for metal binding [10].
Structural Characteristics and Innate Metal Affinity
The chemical structure of chitosan is the foundation of its remarkable biosorbent capabilities. Unlike most polysaccharides, chitosan possesses positively charged amino groups in acidic environments, making it a polycationic polymer [10]. This feature is crucial for its interactions with various metal ions.
The primary functional groups responsible for metal chelation are [11] [10]:
- Amino groups (-NH₂): These are the most reactive sites, capable of forming coordination bonds with metal ions.
- Hydroxyl groups (-OH): These can also participate in metal binding, though to a lesser extent than amino groups.
The proposed mechanisms for metal sorption include:
- Chemisorption: Formation of coordination complexes between metal ions and the amino groups, often following pseudo-second-order kinetics [14] [9].
- Electrostatic attraction: Between protonated amino groups (NH₃⁺) and anionic metal species [9].
The following diagram illustrates the chemical structure of chitosan and its metal-binding sites.
Comparative Performance of Chitosan and Alternative Biosorbents
A wide array of materials has been investigated for their metal sorption capabilities. The following table provides a comparative overview of the adsorption capacities of chitosan and other common biosorbents for various heavy metals, based on data from recent scientific literature.
Table 1: Comparative Adsorption Capacities of Chitosan and Alternative Biosorbents for Heavy Metals
| Adsorbent Material | Target Metal Ions | Reported Adsorption Capacity (mg/g) | Key Advantages | Key Limitations |
|---|---|---|---|---|
| Chitosan (Base Polymer) | Cu²⁺, Cd²⁺, Pb²⁺ [15] | >90% removal efficiency [15] | High innate affinity, biodegradable, renewable [10] | Soluble in strong acids, poor mechanical strength [9] |
| Magnetic Chitosan/CNF-Fe(III) Composite [12] | Cr(VI), Cu(II), Pb(II) | Not specified (high removal efficiency) [12] | Magnetic separation, enhanced stability [12] | Multi-step synthesis required |
| TPP-Crosslinked Magnetic Chitosan (TPP-CMN) [13] | Cd(II), Co(II), Cu(II), Pb(II) | 87.25 - 99.96 mg/g [13] | Fast kinetics (15 min), high capacity, reusable [13] | Surface modification needed |
| Vanillin-Modified Magnetic Chitosan (V-CMN) [13] | Cd(II), Co(II), Cu(II), Pb(II) | 88.75 - 99.89 mg/g [13] | High capacity, reusable [13] | Slightly slower kinetics (30 min) than TPP-CMN [13] |
| Chemically Modified Chitosan Coated Sugarcane Bagasse Ash [16] | Pb(II), Cd(II) | ~12 mg/g [16] | Low-cost composite, uses agricultural waste [16] | Lower capacity than advanced composites |
| Bone Char [14] | Cr(VI), Cd(II), Pb(II) | High for Cr(VI) at low pH [14] | Effective for specific ions like Cr(VI) [14] | Performance varies with bone source and pyrolysis conditions [14] |
| Food Wastes (e.g., Rice Husks, Sugarcane Bagasse) [14] | Cr, Pb | Varies by waste type [14] | Very low cost, waste valorization [14] | Inconsistent composition and adsorption efficiency [14] |
Performance of Nanomagnetic Chitosan Composites
The integration of magnetic nanoparticles (e.g., Fe₃O₄) into chitosan matrices has led to a new class of biosorbents that address several limitations of pure chitosan. The table below details the synthesis and performance of different nanomagnetic chitosan composites, highlighting their comparative efficiency.
Table 2: Synthesis and Performance of Nanomagnetic Chitosan Composites
| Composite Name | Synthesis Method & Modification | Key Characteristics | Optimal Adsorption Conditions | Adsorption Performance |
|---|---|---|---|---|
| M-Ch/CNF-Fe(III) [12] | Blend of chitosan and cellulose nanofiber (1:1) coated with Fe₃O₄ via sol-gel and emulsification [12] | Porous structure, magnetic separation, improved mechanical strength [12] | pH-dependent removal for Cr(VI), Cu(II), Pb(II) [12] | Effective elimination of multiple metals from aqueous solution [12] |
| TPP-CMN [13] | Chitosan-coated Fe₃O4 crosslinked with sodium tripolyphosphate (TPP) [13] | Saturation magnetization: 7.211 emu/g, Surface area: 8.75 m²/g [13] | Fast equilibrium (15 minutes) [13] | Pb(II): 99.96, Co(II): 93.00, Cd(II): 91.75, Cu(II): 87.25 mg/g [13] |
| V-CMN [13] | Chitosan-coated Fe₃O4 modified with vanillin (V) [13] | Saturation magnetization: 7.772 emu/g, Surface area: 6.96 m²/g [13] | Equilibrium in 30 minutes [13] | Pb(II): 99.89, Co(II): 94.00, Cd(II): 92.5, Cu(II): 88.75 mg/g [13] |
The following workflow summarizes the typical journey from conceptualization to application for a magnetic chitosan composite in a research setting.
Experimental Protocols for Key Studies
Synthesis of Magnetic Chitosan Nanocomposites (CMN)
The synthesis of the core-shell magnetic adsorbent is a foundational protocol [13]:
- Synthesis of Fe₃O₄ Nanoparticles: Dissolve anhydrous FeCl₃ and FeSO₄·6H₂O in a 1:1 molar ratio in distilled water. Mix the solutions and stir for 10 minutes.
- Precipitation: Add a 10% NaOH solution dropwise under constant stirring until pH reaches 12.25. Continue stirring at 1000 rpm for 1 hour at 50°C.
- Hydrothermal Treatment: Transfer the homogeneous black solution to a Teflon-lined autoclave and heat at 120°C for 6 hours. Cool to room temperature, filter the black precipitate, and wash with distilled water and ethanol, then dry at 60°C.
- Chitosan Coating: Add the synthesized Fe₃O₄ nanoparticles to a 3% (w/v) chitosan solution (in acetic acid) in a 1:70 (w/v) ratio. Disperse using ultrasonic vibration for 20 minutes.
- Cross-linking: Add a few drops of formaldehyde as a cross-linker. A black gel forms after 4.5 hours. Dry the product at 60°C, then wash with 2% acetic acid and distilled water, and dry again [13].
Surface Modification with Tripolyphosphate (TPP-CMN)
For enhanced stability and performance, surface modification can be performed [13]:
- Suspend the prepared CMN in a 6% aqueous citric acid solution for 18 hours under magnetic stirring.
- Add a sodium tripolyphosphate (TPP) solution dropwise into the yellowish opaque solution.
- Sonicate the mixture for 15 minutes and then stir for an additional 5 hours.
- Filter the resulting whitish solution to recover the TPP-CMN nano-sorbent [13].
Batch Adsorption Experiment Protocol
A standardized batch equilibrium technique is used to evaluate performance [16] [13]:
- Preparation: Prepare stock solutions (e.g., 1000 mg/L) of target metal ions (Pb(II), Cd(II), Cu(II), Cr(VI), etc.) from their salts.
- Parameter Testing: Agitate a fixed dose of the biosorbent (e.g., 0.05–1.0 g) with a known volume (e.g., 100 mL) of metal ion solution in a shaker incubator.
- Variable Parameters: Systematically vary parameters such as initial pH (3.0–8.0), contact time (15–90 min), initial metal concentration (10–250 mg/L), and temperature (28–80°C).
- Analysis: After agitation, separate the adsorbent (via filtration or magnetic separation for magnetic composites). Analyze the residual metal ion concentration in the supernatant using Atomic Absorption Spectroscopy (AAS) or Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES).
- Calculation: Calculate the adsorption capacity at equilibrium (qₑ in mg/g) using the formula:
qₑ = (C₀ - Cₑ) * V / m, where C₀ and Cₑ are the initial and equilibrium concentrations (mg/L), V is the volume of solution (L), and m is the mass of the adsorbent (g) [16] [13].
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 3: Key Reagents and Materials for Chitosan Biosorbent Research
| Reagent/Material | Typical Function in Research | Example Application |
|---|---|---|
| Chitosan | Primary biosorbent material; source of amino functional groups [10] | Base polymer for creating composites and beads [16] |
| FeCl₃·6H₂O / FeSO₄·7H₂O | Precursors for synthesis of magnetic Fe₃O₄ (magnetite) nanoparticles [13] | Creating the magnetic core for easy separation of composites [12] [13] |
| Sodium Tripolyphosphate (TPP) | Cross-linking agent; enhances chemical stability in acidic solutions [13] | Producing TPP-CMN; forms ionic bonds with chitosan amino groups [13] |
| Acetic Acid | Solvent for dissolving chitosan [10] | Preparation of chitosan coating solutions [13] |
| Glutaraldehyde | Cross-linking agent; improves mechanical strength and acid resistance [16] | Cross-linking chitosan in composite beads (ASB-CBs) [16] |
| Sodium Hydroxide (NaOH) | Precipitating agent for Fe₃O₄; pH adjustment in adsorption studies [13] | Precipitation during nanoparticle synthesis; adjusting solution pH [16] [13] |
| Vanillin | Surface modifying agent; introduces additional functional groups [13] | Producing vanillin-modified magnetic chitosan (V-CMN) [13] |
Chitosan's natural abundance, biodegradability, and unique polycationic structure confer a innate and powerful affinity for heavy metal ions, solidifying its status as a premier biosorbent material. While raw chitosan has limitations, the development of nanomagnetic chitosan composites represents a significant advancement, successfully addressing challenges related to separation, stability, and sorption capacity. Comparative data clearly shows that these engineered composites, such as TPP-CMN and V-CMN, achieve superior removal efficiencies for a wide spectrum of toxic metals like Pb(II), Cd(II), and Cu(II), outperforming many alternative materials like raw food wastes or bone char. The integration of magnetic properties not only enhances performance but also aligns with sustainable water treatment goals by enabling easy recovery and reuse. Future research should focus on optimizing synthesis for lower costs, exploring selective modifications for complex wastewater, and scaling up production to facilitate the real-world application of these highly promising composite materials.
The removal of heavy metals from contaminated water sources represents a critical challenge in environmental remediation. Among the various technologies developed, adsorption has emerged as a preferred method due to its simplicity, efficiency, and cost-effectiveness [17]. However, conventional adsorbents often present limitations in recovery and reusability, necessitating complex separation processes that increase operational costs and potential for secondary pollution. The integration of magnetic iron oxides, primarily Fe₃O₄ (magnetite) and γ-Fe₂O₃ (maghemite), into composite materials has revolutionized this field by enabling efficient magnetic separation while enhancing adsorption performance [5] [17]. These magnetic components impart their unique properties to composites, allowing for rapid retrieval using external magnetic fields after the adsorption process is complete, thereby addressing key challenges in practical application of nanoparticle-based water treatment technologies.
The combination of magnetic iron oxides with biopolymers such as chitosan represents a particularly promising approach, merging the excellent adsorption properties of chitosan with the magnetic responsiveness of iron oxides [5] [18]. Chitosan, derived from chitin, offers abundant amino and hydroxyl functional groups that effectively coordinate with heavy metal ions, while the magnetic components facilitate separation and reuse [5] [19]. This comparative analysis examines the performance of various nanomagnetic chitosan composites for heavy metal removal, with focus on their adsorption capacities, separation efficiency, reusability, and implementation in complex wastewater environments.
Performance Comparison of Nanomagnetic Chitosan Composites
The efficacy of nanomagnetic chitosan composites varies significantly based on their structural composition, functionalization methods, and target heavy metals. The table below summarizes the performance characteristics of key composite types documented in recent research.
Table 1: Performance Comparison of Nanomagnetic Chitosan Composites for Heavy Metal Removal
| Composite Type | Target Heavy Metals | Maximum Adsorption Capacity (mg/g) | Equilibrium Time (min) | Magnetic Separation | Reusability Cycles | Key Advantages |
|---|---|---|---|---|---|---|
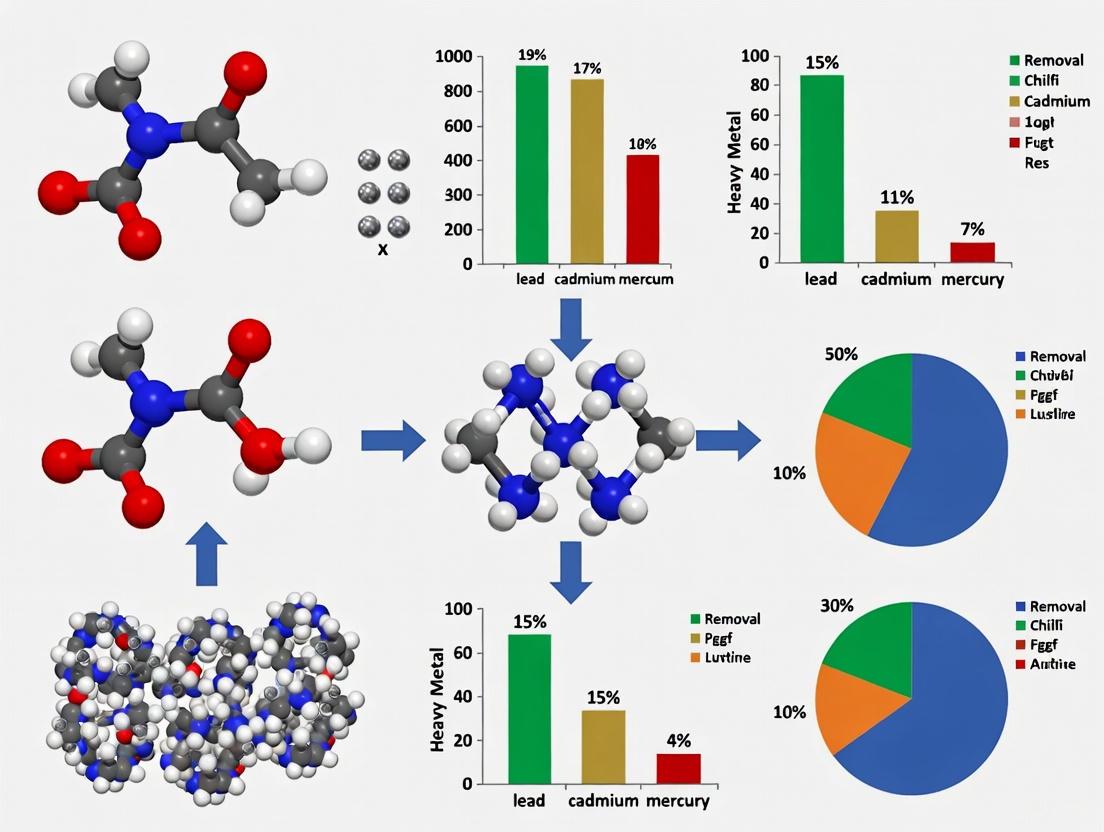
| Magnetic Chitosan Composite (MCC) [5] | Cu(II), Pb(II), Ni(II) | Pb(II): 220.9, Cu(II): 216.8, Ni(II): 108.9 | 120 | 12 emu/g saturation magnetization | Not specified | High versatility for multiple metals, good magnetization |
| Fe₃O₄/Chitosan/Polypyrrole [20] | Cr(VI) | 193.23 | Not specified | Efficient magnetic recovery | 5 cycles (84.32% efficiency) | Excellent acidic stability, good recyclability |
| CS/Fe₃O₄ Nanocomposite [18] | Mixed heavy metals | 100% removal efficiency | 30 | Effective magnetic response | Not specified | Broad-spectrum removal, rapid kinetics |
| Xanthate-Modified Magnetic Composite (XMPC) [19] | Cd(II) | 307 | 120 | Easy magnetic separation | Multiple reuses demonstrated | Exceptional Cd(II) capacity, chemically stable |
| NH₂-Fe₂O₃/CS [21] | Pb(II), Cu(II), Cd(II) | Selective adsorption: Pb(II) > Cu(II) > Cd(II) | 120 | Magnetic recovery feasible | Not specified | Excellent selectivity for Pb(II) |
| Carbonized Chitosan-Fe₃O₄-SiO₂ [22] | Co(II), Ni(II), Cu(II) mixture | 2908.92 (total capacity) | 90 | Magnetic separation enabled | Not specified | Extraordinary capacity for mixed metals |
The data reveals that functionalization strategies profoundly influence composite performance. Xanthate modification [19] yields exceptional cadmium uptake capacity (307 mg/g), while carbonization combined with silica incorporation [22] produces remarkably high total adsorption capacity (2908.92 mg/g) for mixed metal systems. Selectivity patterns also vary significantly, with NH₂-functionalized composites [21] exhibiting preferential adsorption for lead over copper and cadmium, following the trend Pb(II) > Cu(II) > Cd(II).
Table 2: Separation Efficiency and Practical Implementation Characteristics
| Composite Type | Saturation Magnetization | Separation Efficiency | Optimal pH Range | Real-Wastewater Performance | Stability Considerations |
|---|---|---|---|---|---|
| Magnetic Chitosan Composite (MCC) [5] | 12 emu/g | Easy magnetic separation | Not specified | Not tested | Contains ~50 wt% chitosan |
| CS/Fe₃O₄ Nanocomposite [18] | Not specified | Effective magnetic response | Not specified | 100% removal from petroleum water | Green synthesis from plant extract |
| Xanthate-Modified Magnetic Composite (XMPC) [19] | Not specified | Easily magnetically separated | Not specified | Not tested | Improved mechanical stability vs. pure chitosan |
| Fe₃O₄/γ-Fe₂O₃-based Composites [23] | Not specified | Magnetically separable | Varied with MOF component | 89-95% dye degradation | MOF framework stability |
| Carbonized Chitosan-Fe₃O₄-SiO₂ [22] | Not specified | Easy magnetic separation | pH = 9 | Not tested | Green synthesis from biowaste |
Magnetic separation capability is consistently demonstrated across composite types, with saturation magnetization values around 12 emu/g sufficient for effective recovery [5]. Composites tested in real wastewater environments, such as petroleum water [18], show promising performance with complete heavy metal removal within 30 minutes, demonstrating practical potential beyond laboratory conditions.
Experimental Protocols and Methodologies
Synthesis Approaches
Magnetic Chitosan Composite (MCC) Preparation [5]: The synthesis involves embedding pre-formed magnetite/maghemite nanoparticles within a chitosan matrix. Chitosan is typically dissolved in dilute acetic acid solution, followed by addition of iron oxide nanoparticles under mechanical stirring. The composite is then precipitated using alkaline solution, washed, and dried. Characterization through thermogravimetric analysis (TGA) confirms approximately 50 wt% chitosan content, while zeta potential measurements determine an isoelectric point of pH 8-8.5, indicating favorable conditions for cation adsorption.
Green Synthesis of CS/Fe₃O₄ Nanocomposite [18]: This environmentally benign approach utilizes Laurus nobilis leaf extract for bioreduction and stabilization. Fe₃O₄ nanoparticles are first synthesized using the aqueous leaf extract, then coated with chitosan extracted from shrimp shells. The spherical nanoparticles exhibit average sizes of 21.3 nm for Fe₃O₄ and 28 nm for the composite, with optical bandgap energies of 2.62 eV and 1.81 eV, respectively, indicating enhanced photocatalytic potential in the composite form.
Xanthate-Modified Magnetic Composite (XMPC) Fabrication [19]: This multi-step synthesis begins with creation of magnetic Fe₃O₄@SiO₂ core-shell nanoparticles via co-precipitation and tetraethyl orthosilicate (TEOS) hydrolysis. The magnetic particles are then incorporated into a polyvinyl alcohol and chitosan matrix, followed by xanthate modification using carbon disulfide under alkaline conditions. This introduction of xanthate groups significantly enhances heavy metal binding capacity through formation of stable complexes.
Carbonized Chitosan-Fe₃O₄-SiO₂ Synthesis [22]: This novel green nanocomposite preparation involves carbonizing chitosan as a precursor material, then functionalizing with magnetite (Fe₃O₄) and silica (SiO₂) extracted from sugarcane bagasse. The carbonization enhances porosity and stability, while silica improves heavy metal adsorption capacity and magnetite enables magnetic separation. The composite demonstrates exceptional adsorption capacity for mixed metal systems.
Adsorption Experiment Methodologies
Batch adsorption experiments follow standardized protocols across studies [5] [20] [19]. Typically, a predetermined amount of composite is added to heavy metal solutions at controlled pH, temperature, and agitation speed. Samples are collected at time intervals, separated magnetically, and the supernatant analyzed for residual metal concentration using techniques such as inductively coupled plasma mass spectrometry (ICP-MS) or atomic absorption spectroscopy.
Kinetic and Isotherm Modeling [5] [20] [19]:
- Pseudo-second-order kinetics commonly best describe adsorption processes, indicating chemisorption as the rate-limiting step.
- Langmuir isotherm models frequently provide better fit than Freundlich models, suggesting monolayer adsorption on homogeneous surfaces.
- Thermodynamic studies consistently show spontaneous and endothermic processes for effective composites.
Competitive Adsorption Studies [21]: In ternary metal systems (Pb(II), Cu(II), Cd(II)), experiments reveal selective adsorption patterns. Composites like NH₂-Fe₂O₃/CS exhibit preferential adsorption following the order Pb(II) > Cu(II) > Cd(II), with significantly higher lead removal rates attributable to greater binding affinity and possibly coordination geometry preferences.
Characterization Techniques
Comprehensive materials characterization employs multiple analytical methods:
- Electron Microscopy (SEM/TEM): Reveals surface morphology, particle size, and dispersion [18] [21].
- FT-IR Spectroscopy: Identifies functional groups and confirms successful modification [20] [21].
- X-ray Diffraction (XRD): Determines crystallinity and phase composition of magnetic components [18] [21].
- Vibrating Sample Magnetometry (VSM): Quantifies magnetic properties and separation potential [5].
- BET Surface Area Analysis: Measures specific surface area and pore characteristics [21].
- Thermogravimetric Analysis (TGA): Assesses thermal stability and composite composition [5].
The following workflow diagram illustrates the typical experimental process from synthesis to application:
Diagram 1: Experimental workflow for developing and evaluating nanomagnetic chitosan composites, covering synthesis, characterization, and application phases.
Mechanisms of Adsorption and Separation
Heavy Metal Capture Mechanisms
The exceptional adsorption capacity of nanomagnetic chitosan composites stems from multiple simultaneous mechanisms:
Coordination and Complexation [5] [21]: The amino (-NH₂) and hydroxyl (-OH) groups of chitosan serve as electron donors, forming coordinate covalent bonds with heavy metal ions. This chemisorption process is confirmed by pseudo-second-order kinetic models [19]. In NH₂-functionalized composites [21], the introduction of additional amino groups further enhances metal coordination capability.
Electrostatic Interactions [20]: Protonation of amino groups in acidic media creates positive surfaces that attract anionic metal species through Coulombic forces. For Cr(VI) removal, this mechanism predominates at low pH, with efficiency decreasing as pH increases due to reduced protonation.
Chemical Reduction [20]: Some composites, particularly those with polypyrrole components, facilitate reduction of toxic Cr(VI) to less hazardous Cr(III), enhancing removal through precipitation and coordination of the reduced species.
Ion Exchange [22]: Functional groups such as carboxyl and hydroxyl can exchange protons or other ions for heavy metal cations in solution, contributing to overall uptake capacity.
The following diagram illustrates the primary adsorption mechanisms at the composite-water interface:
Diagram 2: Multifunctional adsorption mechanisms in nanomagnetic chitosan composites, culminating in magnetic separation after metal capture.
Magnetic Separation Mechanism
The separation process leverages the inherent magnetic properties of Fe₃O₄ and γ-Fe₂O₃ components [5] [17]. When an external magnetic field is applied, the magnetic moments within the nanoparticles align, generating a net attraction force that exceeds Brownian motion and fluid drag forces. This enables rapid segregation of particle-bound heavy metals from treated water. Composites with saturation magnetization values of approximately 12 emu/g demonstrate efficient separation capabilities [5], with higher values further improving recovery efficiency.
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 3: Essential Research Reagents and Materials for Nanomagnetic Chitosan Composite Studies
| Reagent/Material | Function in Research | Application Examples |
|---|---|---|
| Chitosan | Primary biopolymer matrix providing adsorption sites | Source of amino and hydroxyl groups for metal coordination [5] [18] [19] |
| Fe₃O₄/γ-Fe₂O₃ Nanoparticles | Magnetic component enabling separation | Core magnetic material for composite recovery [5] [18] [23] |
| Polyvinyl Alcohol (PVA) | Polymer additive enhancing mechanical stability | Improves film formation and durability in composites [19] |
| Tetraethyl Orthosilicate (TEOS) | Silica source for core-shell structures | Creates protective SiO₂ layer on magnetic nanoparticles [19] |
| Glutaraldehyde | Crosslinking agent for chitosan | Enhances chemical stability and reusability [21] |
| Xanthating Agents (CS₂/NaOH) | Introduction of sulfur-containing functional groups | Enhances heavy metal chelation, particularly for soft metals [19] |
| Polyvinylpyrrolidone (PVP) | Stabilizing agent for nanoparticles | Prevents aggregation and improves dispersion [24] |
| Aminopropyltriethoxysilane (APTES) | Amino-functionalization agent | Introduces additional amine groups for enhanced metal binding [25] |
| Plant Extracts (e.g., Laurus nobilis) | Green synthesis reducing/ stabilizing agents | Eco-friendly alternative to chemical reducing agents [18] |
The integration of Fe₃O₄ and γ-Fe₂O₄ into chitosan composites represents a significant advancement in heavy metal removal technologies, successfully addressing the critical challenge of nanoparticle recovery while enhancing adsorption performance through synergistic effects. Comparative analysis reveals that while base magnetic chitosan composites offer good all-around performance [5], strategically functionalized composites demonstrate specialized superiority: xanthate-modified variants excel in cadmium removal [19], hyperbranched composites with PAMAM dendrimers show remarkable selectivity [25], and carbonized chitosan composites achieve extraordinary capacity for mixed metal systems [22].
The magnetic revolution in water treatment continues to evolve, with current research focusing on enhancing selectivity for specific heavy metals, improving stability in extreme pH conditions, and developing scalable green synthesis methods. The optimal composite selection depends heavily on the specific application requirements, including target metals, wastewater composition, and operational constraints. As these technologies transition from laboratory validation to real-world implementation, nanomagnetic chitosan composites stand poised to make substantial contributions to global efforts in water purification and heavy metal pollution mitigation.
In the pursuit of advanced water purification technologies, nanomagnetic chitosan composites have emerged as a leading class of materials for removing heavy metals from contaminated water. The performance of these materials hinges on a fundamental principle of nanoscience: the strategic optimization of surface area and active sites through precise engineering at the nanoscale. By integrating magnetic nanoparticles with the biopolymer chitosan and various functional modifiers, researchers create sophisticated architectures that maximize the availability of binding sites for heavy metal ions while incorporating practical features for material recovery and reuse.
This guide provides an objective comparison of various nanomagnetic chitosan composites, evaluating their removal efficiency for different heavy metals based on experimental data from recent scientific studies. We examine how different modification strategies—including the incorporation of cyclodextrin, lignin, polyvinyl alcohol, silica, and other functional groups—synergistically enhance the inherent properties of chitosan to create composites with superior adsorption capabilities.
Comparative Performance of Nanomagnetic Chitosan Composites
The efficacy of nanomagnetic chitosan composites varies significantly based on their specific composition, structure, and target pollutants. The table below summarizes the removal performance of various composites for different heavy metals, based on experimental data from recent studies.
Table 1: Comparison of heavy metal removal by different nanomagnetic chitosan composites
| Composite Type | Target Pollutant | Maximum Adsorption Capacity (mg/g) | Optimal pH | Removal Efficiency | Reference |
|---|---|---|---|---|---|
| Chitosan-Magnetite Strip | Cr(VI) | - | - | 92.33% | [26] |
| S1@Chitosan (Na-Fe Silicate) | Cd(II) | 389.11 | 7.5 | - | [27] |
| PVA-Modified Chitosan | Cu(II) | 303.29 | - | - | [28] |
| PVA-Modified Chitosan | Ni(II) | 209.08 | - | - | [28] |
| PVA-Modified Chitosan | Zn(II) | 173.39 | - | - | [28] |
| Chitosan-Lignin Biocomposite | Cr(VI) | 72.61 | 2.0 | - | [29] |
| Fe₃O₄@Si-OH@CS | Cr(VI) | - | 2.5 | High | [30] |
| Fe₃O₄@Si-OH@CS | As, Hg, Se | - | - | High | [30] |
Table 2: Surface characteristics and experimental conditions of selected composites
| Composite Type | Surface Area (m²/g) | Contact Time (min) | Initial Concentration | Temperature (K) | Reference |
|---|---|---|---|---|---|
| S1@Chitosan | 30.94 | 50 | Varying | 298 | [27] |
| Nanomagnetite/Chitosan (CSG) | 878.7 | 720 (adsorption) | Varying | Ambient | [31] |
| PVA-Modified Chitosan | 9.36 | - | Multi-metal system | Varied | [28] |
Fundamental Mechanisms of Synergistic Enhancement
Surface Area Expansion
The incorporation of magnetite nanoparticles and porous additives dramatically increases the available surface area of chitosan composites. For instance, while native chitosan has limited surface area, composites like nanomagnetite/chitosan (CSG) demonstrate remarkably high surface areas of up to 878.7 m²/g [31]. This expansion creates more opportunities for metal ions to encounter and bind to active sites. The porous structure of composites such as chitosan-lignin provides numerous accessible binding sites, facilitating higher adsorption capacities for both dyes and heavy metals [29].
Active Site Optimization
Nanoscale engineering enhances not only the quantity but also the quality and accessibility of active sites:
- Amino Group Utilization: Chitosan's primary amino groups serve as key coordination sites for metals [32]. Composite formation protects these groups while increasing their accessibility.
- Multi-functional Group Integration: Modifiers like cyclodextrin introduce additional functional groups that create diverse binding environments [33]. Silanol groups (Si-OH) in silica-modified composites provide additional adsorption sites through ion exchange and complexation [30].
- Synergistic Binding: In chitosan-lignin composites, the combination of amino, hydroxyl, and phenolic groups enables multiple simultaneous interactions with metal ions, leading to superior removal efficiency compared to either component alone [29].
Magnetic Separation Enhancement
The integration of Fe₃O₄ nanoparticles provides ferrimagnetism, enabling efficient recovery of spent adsorbents using external magnetic fields [31] [26] [30]. This practical advantage maintains the high surface area and active site availability while facilitating material reuse—a critical consideration for practical applications.
Experimental Protocols and Methodologies
Synthesis Methods
Co-precipitation for Magnetic Composites
The most common synthesis approach for nanomagnetic chitosan composites involves co-precipitation, as exemplified by the Fe₃O₄@Si-OH@CS preparation [30]:
- Magnetic Core Formation: FeCl₃·6H₂O and FeSO₄·7H₂O dissolved in deionized water at 2:1 molar ratio, followed by addition of NH₃·H₂O at 80°C with continuous stirring for 40 minutes
- Silica Functionalization: Magnetic fluid dispersed in ethanol/water mixture, followed by addition of NH₃·H₂O and ethyl orthosilicate with agitation at 60°C for 4.5 hours
- Chitosan Coating: Fe₃O₄@Si-OH nanoparticles dispersed in chitosan solution (1%) with glutaraldehyde as crosslinker, stirred at 60°C for 30 minutes
- Product Recovery: Magnetic separation, washing with acetic acid (3%), and drying at 80°C
Similar co-precipitation methods are employed for other magnetic chitosan composites, with variations in precursor ratios and functionalization steps [33] [26].
Composite Formation without Magnetic Core
For non-magnetic composites like chitosan-lignin:
- Chitosan Solution Preparation: Chitosan dissolved in acetic acid (0.1 mol/L) with continuous stirring for 24 hours
- Lignin Integration: Predetermined amount of lignin dissolved separately, then gradually added to chitosan solution
- Film Formation: Solution cast onto glass supports, dried at room temperature, peeled, and converted to base form
- Powder Preparation: Dried composite ground, washed with distilled water, vacuum filtered, and dried at 100°C [29]
Adsorption Experiments
Standard batch adsorption experiments typically involve:
- Solution Preparation: Stock solutions of heavy metals (typically 500-1000 mg/L) prepared using salts like K₂Cr₂O₇ for Cr(VI) or Cd(NO₃)₂·4H₂O for Cd(II)
- pH Adjustment: Solution pH adjusted using NaOH or HCl (0.1 M) to optimal conditions
- Adsorption Process: Composite added to metal solution at specific dosage, with agitation at constant speed and temperature
- Sampling and Analysis: Samples collected at time intervals, centrifuged, and supernatant analyzed via AAS or UV-Vis spectrophotometry [27] [29] [28]
Analytical Methods
Comprehensive characterization employs multiple techniques:
- Surface Area and Porosity: BET analysis using N₂ adsorption-desorption at 77K [27] [31]
- Structural Confirmation: FT-IR spectroscopy to identify functional groups [33] [26]
- Crystallinity Assessment: XRD analysis to determine crystalline phases [31] [26]
- Morphological Examination: SEM and TEM for surface morphology and particle size [33] [31]
- Magnetic Properties: VSM to confirm magnetic behavior [31] [26]
- Elemental Composition: EDX for elemental mapping and confirmation [33] [27]
Visualization of Synthesis and Mechanism
Diagram 1: Synthesis pathway and adsorption mechanism of nanomagnetic chitosan composites
The Researcher's Toolkit: Essential Materials and Reagents
Table 3: Key research reagents for synthesizing and testing nanomagnetic chitosan composites
| Reagent/Material | Function/Application | Examples from Literature |
|---|---|---|
| Chitosan | Primary biopolymer matrix providing amino groups for metal coordination | Medium molecular weight [33]; Low viscosity < 200 mPa·s [30]; High molecular weight, 90% deacetylation [28] |
| FeCl₃·6H₂O and FeSO₄·7H₂O | Precursors for magnetite (Fe₃O₄) nanoparticle synthesis via co-precipitation | Used in 2:1 molar ratio [26] [30] |
| NH₃·H₂O (Ammonium Hydroxide) | Precipitation agent for magnetite formation; catalyst for silica condensation | 25-28% solution [30] |
| Ethyl Orthosilicate | Silicon precursor for creating silanol-functionalized surfaces | [30] |
| Glutaraldehyde | Crosslinking agent for chitosan stabilization | 50% solution [30] |
| Polyvinyl Alcohol (PVA) | Polymer modifier enhancing mechanical strength and surface properties | [28] |
| Lignin | Biopolymer additive introducing phenolic groups for enhanced metal binding | 97% purity [29] |
| β-Cyclodextrin | Macrocyclic modifier creating host-guest complexes and additional binding sites | Grafted onto magnetic chitosan [33] |
| Heavy Metal Salts | Target pollutants for adsorption testing | K₂Cr₂O₇ for Cr(VI) [26] [29]; Cd(NO₃)₂·4H₂O for Cd(II) [27] |
The comparative analysis of nanomagnetic chitosan composites reveals a clear structure-property-performance relationship. Composites with higher surface areas and optimized active sites consistently demonstrate superior heavy metal removal capabilities. The integration of magnetic components enables practical recovery and reuse, while chemical modifiers like silanol groups, lignin, and cyclodextrin introduce complementary binding mechanisms that enhance both capacity and selectivity.
The experimental data indicate that composite performance depends significantly on the target metal ion and solution conditions. Cr(VI) removal is most efficient under acidic conditions, while Cd(II) removal occurs optimally near neutral pH. Multi-metal systems present competitive adsorption scenarios where composite affinity varies across different metal ions.
These findings underscore the importance of tailored composite design for specific application requirements. Future research directions should focus on developing selective composites for complex multi-pollutant systems, improving regeneration efficiency, and scaling up synthesis protocols for practical water treatment applications.
The escalating contamination of aquatic environments by heavy metals constitutes a critical threat to global ecosystems and human health, driving an urgent need for effective and sustainable remediation strategies [34]. Among the various technologies developed, adsorption is widely regarded as a superior method due to its simplicity, cost-effectiveness, and high efficiency [35]. Within this domain, nanomagnetic chitosan composites have emerged as a frontier adsorbent class, synergizing the exceptional metal-binding capacity of chitosan—a natural, biodegradable polymer—with the facile separation capabilities of magnetic nanoparticles [9]. The research field surrounding these composites is experiencing explosive growth, characterized by vigorous international collaboration and a high rate of scientific output. This review employs a bibliometric analysis to map this dynamic landscape, providing a systematic comparison of the removal efficiencies of various nanomagnetic chitosan composites for heavy metals, supported by experimental data and mechanistic insights.
Bibliometric Analysis of the Research Field
A quantitative examination of scientific literature reveals the vigorous evolution of research into chitosan-based materials for water treatment. A systematic review analyzing 1,855 publications from 2005 to 2025 documented an annual growth rate of 21.52% and an average of 32.7 citations per article, underscoring the field's strong scientific vitality and impact [34]. A separate bibliometric study focusing on 1,690 documents from 2014 to 2024 found a similarly robust annual growth rate of 16.86% and an average of 32.12 citations per document, confirming the sustained and rising interest in this area [36] [37].
Geographically, research production is heavily concentrated in Asia. China, India, and Iran collectively contribute nearly 60% of the global scientific output on chitosan composites for wastewater treatment [34] [36]. This high level of productivity is complemented by significant international cooperation, with about 26.78% of publications involving cross-border collaborations, highlighting the global recognition of the water pollution challenge and the concerted effort to address it [36].
The intellectual focus of the field, as revealed by keyword network analysis, is centered on several key themes. Predominant among these are "adsorption mechanisms" and "composite functionalization," with a strong and growing emphasis on "magnetic hybrids" and "nano-reinforced composites" designed for the targeted removal of specific pollutants [36] [9].
Comparative Removal Efficiency of Nanomagnetic Chitosan Composites
The performance of nanomagnetic chitosan composites varies significantly based on their specific constituents and synthesis methods. The table below provides a comparative overview of the removal efficiencies of different composite types for various heavy metals, as reported in recent literature.
Table 1: Comparison of Removal Efficiencies for Various Nanomagnetic Chitosan Composites
| Composite Name | Target Heavy Metal(s) | Reported Removal Efficiency | Optimal pH | Key Findings/Mechanism |
|---|---|---|---|---|
| Chitosan/g-HNTs@ZnγFe₃O₄ [38] | Cr(III), Fe(III), Mn(II) | 95.2%, 99.06%, 87.1% | 9.0 | Superior performance attributed to multiple functional groups and coordination bonding. |
| M-Ch/CNF-Fe(III) [12] | Cr(VI), Cu(II), Pb(II) | High removal demonstrated | Varied (1-8) | Porous structure effective for multiple metals; adsorption fitted Langmuir isotherm. |
| Chitosan-Magnetic Nanocomposites [34] | Various dyes and toxic metals | Adsorption capacities > 400 mg g⁻¹ | Not Specified | Engineered nanostructure provides high surface area and enhanced porosity. |
| Magnetic Chitosan (General Review) [9] | Pb, Hg, Cr, Cd, Cu, As | Outstanding adsorption performance | Varied | Good adaptability in real industrial wastewater and multi-metal systems. |
The data indicates that composite materials often leverage synergistic effects between their components. For instance, the incorporation of halloysite nanotubes and zinc-doped magnetite (Chitosan/g-HNTs@ZnγFe₃O₄) creates a quaternary nanocomposite with a dense array of functional groups, leading to exceptional removal efficiencies exceeding 95% for certain ions like Fe(III) [38]. Similarly, composites that integrate biopolymers like cellulose (M-Ch/CNF-Fe(III)) benefit from the combined reactive sites of both polymers and the magnetic properties of Fe₃O₄, enabling effective removal of oxyanions like Cr(VI) and cations like Pb(II) and Cu(II) across a wide pH range [12].
Detailed Experimental Protocols and Workflows
To ensure the reproducibility of the high-performance results cited, this section outlines the standard experimental protocols for synthesizing a representative composite and evaluating its adsorption efficacy.
Synthesis of a Magnetic Chitosan Nanocomposite
A common and effective method for preparing magnetic chitosan composites is the chemical co-precipitation technique [38] [12]. The following workflow details the synthesis of a magnetic chitosan/cellulose-Fe(III) composite [12]:
Key Reagents and Functions:
- FeCl₃·6H₂O: Iron precursor for generating magnetic Fe₃O₄ nanoparticles.
- Sodium Acetate: Serves as an alkaline agent to facilitate precipitation.
- Ethylene Glycol: Acts as a solvent and reducing agent.
- Chitosan: Provides the primary matrix with amino and hydroxyl functional groups for metal binding.
- Cellulose Nanofiber (CNF): Enhances porosity and mechanical strength, and provides additional hydroxyl groups.
- 1-Butyl-3-methylimidazolium Chloride (Ionic Liquid): Functions as a green solvent to disperse and homogenize the polymer blend.
- Tween 80: A surfactant used to stabilize the emulsion during synthesis.
Batch Adsorption Experiment Protocol
The evaluation of adsorption performance follows a standardized batch protocol to determine optimal conditions and capacities [38] [12]. The workflow below is generic and can be applied to test any composite for various heavy metals.
Critical Parameters & Isotherm/Kinetic Modeling:
- Parameters: The effects of pH (1.0-9.0), adsorbent dosage (0.05-1.0 g/L), contact time (15-90 min), initial metal concentration, and temperature (28-80°C) are systematically investigated [38] [12].
- Isotherm Models: Experimental data is fitted to models like Langmuir (assuming monolayer adsorption) and Freundlich (assuming heterogeneous surface) to understand the adsorption distribution at equilibrium [38] [12].
- Kinetic Models: Data from time-series experiments is fitted to pseudo-first-order and pseudo-second-order models to elucidate the adsorption rate and mechanism [38] [12].
The Scientist's Toolkit: Essential Research Reagents and Materials
The development and testing of nanomagnetic chitosan composites rely on a core set of reagents and materials. The following table lists these essential components and their functions in synthesis and application.
Table 2: Key Research Reagent Solutions for Composite Development
| Reagent/Material | Function in Research & Development | Examples from Literature |
|---|---|---|
| Chitosan | Primary biopolymer matrix; provides amino (-NH₂) and hydroxyl (-OH) groups for metal coordination/chelation. | Derived from shrimp, crab shells; backbone of all composites [34] [39]. |
| Magnetic Precursors (FeCl₃, FeSO₄) | Source of iron for in-situ precipitation of magnetic nanoparticles (Fe₃O₄, γ-Fe₂O₃) within the polymer matrix. | FeCl₃·6H₂O used in co-precipitation [38] [12]. |
| Cross-linkers (Glutaraldehyde, Epichlorohydrin) | Stabilize chitosan hydrogels, improve mechanical strength and chemical resistance in aqueous environments. | Glutaraldehyde used in AUCH-G hydrogels for alkaline conditions [40]. |
| Natural Reinforcements (Halloysite Nanotubes, Cellulose Nanofiber) | Enhance surface area, porosity, mechanical strength, and introduce additional sorption sites. | Halloysite Nanotubes (HNTs) [38]; Cellulose Nanofiber (CNF) [12]. |
| Metal Salts (K₂Cr₂O₇, Pb(NO₃)₂, CuSO₄) | Used to prepare standard solutions for simulating heavy metal contamination in adsorption experiments. | K₂Cr₂O₇, CuSO₄·5H₂O, Pb(NO₃)₂ used in batch tests [12]. |
The global research landscape for nanomagnetic chitosan composites is marked by rapid growth and intense international focus, primarily driven by the pressing need for advanced water purification technologies. Bibliometric evidence confirms the field's strong scientific vitality and its identification as a high-priority research area. Quantitative comparisons reveal that these composites can achieve remarkable removal efficiencies, often exceeding 90% or even 95% for critical heavy metals like Cr(III), Fe(III), and Pb(II). Their performance is heavily influenced by the specific design of the composite, which tailors properties such as surface area, porosity, and functional group density. The reproducibility of these high-performance results is ensured by well-established experimental protocols for synthesis and evaluation. As research progresses, future efforts are directed toward optimizing preparation and modification processes, deepening the understanding of interactions in complex multi-metal systems, and enhancing the regeneration ability of these promising materials for sustainable, real-world application [9].
Synthesis and Performance: Building and Testing Effective Composites
The removal of heavy metals from wastewater is a critical environmental challenge due to their non-biodegradable nature and toxic effects on living organisms [41] [42]. Among various treatment technologies, adsorption has gained significant attention for its efficiency, operational simplicity, and cost-effectiveness [41]. In recent years, nanomagnetic chitosan composites have emerged as promising adsorbents, combining the excellent metal-binding capacity of chitosan with the facile magnetic separation capability of iron oxides [5] [6].
Chitosan, a natural biopolymer derived from chitin, possesses amino and hydroxyl functional groups that effectively coordinate with heavy metal ions [41]. However, its practical application is limited by low mechanical strength, solubility in acidic media, and difficult separation after use [41]. These limitations can be overcome by combining chitosan with magnetic nanoparticles and employing appropriate synthesis methods to enhance stability and performance [5] [43].
The comparative removal efficiency of nanomagnetic chitosan composites is fundamentally governed by their synthesis route. This review comprehensively examines three core preparation techniques—co-precipitation, cross-linking, and hydrothermal synthesis—focusing on their methodological principles, resultant composite characteristics, and performance in heavy metal removal applications for researcher and scientist audiences.
Methodological Principles and Experimental Protocols
Co-precipitation
Principles: Co-precipitation is a straightforward and widely used method for synthesizing magnetic chitosan composites. This technique involves the simultaneous precipitation of magnetic iron oxides (typically magnetite, Fe₃O₄, or maghemite, γ-Fe₂O₃) and their incorporation into a chitosan matrix under alkaline conditions [43]. The process leverages the in-situ formation of magnetic nanoparticles within the chitosan solution, allowing for direct integration of both components.
Experimental Protocol (Chitosan-Nickel Ferrite Composite):
- Step 1: Dissolve ferric chloride (FeCl₃·6H₂O) and nickel chloride (NiCl₂·6H₂O) in deionized water at a molar ratio of 2:1 (Fe:Ni) [43].
- Step 2: Prepare a 1% (w/v) chitosan solution by dissolving chitosan powder in acetic acid solution (1% v/v) with continuous stirring until a clear solution is obtained [43].
- Step 3: Mix the chitosan solution with the metal salts solution under constant stirring at 60°C for 30 minutes to ensure homogeneous distribution [43].
- Step 4: Adjust the pH to approximately 11 using sodium hydroxide (NaOH) solution (0.2 M) to initiate precipitation. The addition of a small amount of oleic acid (0.01 mL) as a surfactant can help control particle growth and prevent aggregation [43].
- Step 5: Heat the mixture to 80°C and maintain with stirring for 6 hours to complete the reaction and aging process [43].
- Step 6: Separate the resulting dark brown precipitates by centrifugation, wash thoroughly with distilled water and ethanol to remove impurities, and dry at room temperature for 20 hours [43].
- Step 7: For enhanced crystallinity, calcine the product at 600°C for 3 hours in a tube furnace [43].
Cross-linking
Principles: Cross-linking method focuses on strengthening the chitosan matrix by creating covalent bonds between polymer chains using cross-linking agents. This approach enhances the chemical stability and mechanical robustness of chitosan composites, preventing their dissolution in acidic environments and improving reusability [41]. Magnetic components can be incorporated either during or after the cross-linking process.
Experimental Protocol (Magnetic Chitosan Microspheres):
- Step 1: Prepare a chitosan solution (2-4% w/v) in dilute acetic acid (2% v/v) and filter to remove any undissolved particles [44].
- Step 2: Synthesize or disperse pre-formed magnetic nanoparticles (e.g., Fe₃O₄) in the chitosan solution under ultrasonication for 30 minutes to achieve homogeneous dispersion [44].
- Step 3: Add the cross-linking agent (commonly glutaraldehyde at 1-2% v/v concentration) dropwise to the mixture under continuous stirring. The amino groups of chitosan react with aldehyde groups to form Schiff base linkages [44].
- Step 4: For microsphere formation, drip the mixture into a coagulation bath containing sodium hydroxide (NaOH) solution (0.1-0.5 M) or tripolyphosphate (TPP) solution (1-2% w/v) using a syringe pump [44].
- Step 5: Maintain the microspheres in the coagulation bath for 1-2 hours to complete the cross-linking and solidification process.
- Step 6: Collect the magnetic microspheres by filtration or magnetic separation, wash extensively with distilled water until neutral pH, and dry at 40-50°C [44].
Hydrothermal Synthesis
Principles: Hydrothermal synthesis utilizes elevated temperature and pressure in a sealed autoclave to facilitate the crystallization and assembly of magnetic chitosan composites. This method typically produces materials with enhanced crystallinity, controlled morphology, and high purity [45] [46]. The method allows for precise control over particle size and structure by adjusting reaction parameters.
Experimental Protocol (Magnetic Chitosan/Cellulose Hybrid Microspheres):
- Step 1: Dissolve chitosan and cellulose in NaOH/urea aqueous solution to form a homogeneous polymer solution [44].
- Step 2: Disperse pre-synthesized magnetic nanoparticles (e.g., γ-Fe₂O₃) in the polymer solution under vigorous stirring [44].
- Step 3: Transfer the mixture to a Teflon-lined stainless steel autoclave, filling 70-80% of its capacity to allow for pressure development [46].
- Step 4: Heat the autoclave to 120-180°C and maintain for 4-24 hours, depending on the desired crystallinity and particle size [46].
- Step 5: Allow the autoclave to cool naturally to room temperature.
- Step 6: Collect the resulting product by magnetic separation or centrifugation, wash with distilled water and ethanol, and dry at 60°C [44] [46].
Comparative Analysis of Composite Properties
The synthesis method significantly influences the structural characteristics, magnetic properties, and functional performance of nanomagnetic chitosan composites. The table below summarizes the key properties achievable through each method.
Table 1: Characteristics of Nanomagnetic Chitosan Composites Prepared by Different Methods
| Property | Co-precipitation | Cross-linking | Hydrothermal Synthesis |
|---|---|---|---|
| Particle Size (nm) | 40-100 [43] | 500-1000 [44] | 25-50 [44] |
| Surface Area (m²/g) | Moderate (50-150) | Variable (20-100) | High (200-400) [45] |
| Magnetization (emu/g) | 17-40 [43] | 10-25 | 12-20 [5] [6] |
| Crystallinity | Moderate | Low to Moderate | High [46] |
| Structural Control | Limited | Good for morphology | Excellent for morphology & size [46] |
| Processing Time | 6-12 hours [43] | 2-6 hours | 4-24 hours [46] |
| Scale-up Potential | Excellent | Good | Moderate |
| Equipment Cost | Low | Low | High (autoclave required) |
Structural and Morphological Characteristics
Co-precipitation typically yields composites with particle sizes ranging from 40-100 nm, as confirmed by TEM analysis of chitosan-nickel ferrite composites [43]. The process generally produces aggregates unless surfactants are employed to control particle growth.
Cross-linking method often results in larger particles or microspheres (500-1000 nm) with porous structures, suitable for column-based applications [44]. The cross-linking density significantly influences the swelling behavior and mechanical stability.
Hydrothermal synthesis enables the production of composites with controlled morphology and smaller particle sizes (25-50 nm) due to the accelerated reaction kinetics under high temperature and pressure conditions [44]. This method typically produces materials with higher crystallinity and more uniform particle size distribution compared to other methods [46].
Magnetic Properties
The saturation magnetization of composites determines their responsiveness to external magnetic fields for separation. Co-precipitation synthesized chitosan-nickel ferrite composites exhibit magnetization values of 40.67 emu/g for bare NiFe₂O₄ and 17.34 emu/g after chitosan coating [43]. Similarly, magnetic chitosan composites (MCC) prepared through related methods show saturation magnetization of approximately 12 emu/g, sufficient for efficient magnetic separation [5] [6].
Performance in Heavy Metal Removal
The efficacy of nanomagnetic chitosan composites in removing heavy metals from aqueous solutions varies significantly with the synthesis method, which influences the availability of active sites, diffusion pathways, and metal-binding kinetics.
Table 2: Heavy Metal Removal Performance of Nanomagnetic Chitosan Composites
| Heavy Metal | Synthesis Method | Adsorption Capacity (mg/g) | Optimum pH | Equilibrium Time (min) | Reference |
|---|---|---|---|---|---|
| Pb(II) | Cross-linking/MCC | 220.9 | 5-6 | 120 | [5] [6] |
| Cu(II) | Cross-linking/MCC | 216.8 | 5-6 | 120 | [5] [6] |
| Ni(II) | Cross-linking/MCC | 108.9 | 5-6 | 120 | [5] [6] |
| Cu(II) | Co-precipitation | 89-125 | 5-6 | 90-180 | [41] |
| Pb(II) | Co-precipitation | 110-180 | 5-6 | 90-180 | [41] |
| Various | Hydrothermal | 150-250 (estimated) | 5-7 | 60-120 | [44] |
Adsorption Capacity and Kinetics
Composites prepared by cross-linking demonstrate exceptional adsorption capacities for heavy metals, with reported values of 220.9 mg/g for Pb(II), 216.8 mg/g for Cu(II), and 108.9 mg/g for Ni(II) [5] [6]. The cross-linked structure provides mechanical stability while maintaining accessibility to functional groups.
Co-precipitation synthesized composites show moderately high adsorption capacities (89-250 mg/g for Cu(II) and Pb(II), depending on specific modifications) [41]. The adsorption process typically follows pseudo-second-order kinetics, indicating chemisorption as the rate-limiting step [5].
Hydrothermal synthesis produces composites with estimated capacities of 150-250 mg/g, attributed to their high surface area and enhanced crystallinity [44] [45]. The efficient incorporation of magnetic components and chitosan matrix under hydrothermal conditions creates hierarchically porous structures conducive to metal uptake.
pH Dependence and Regeneration
The adsorption of heavy metals onto nanomagnetic chitosan composites is highly pH-dependent, with optimal performance observed in slightly acidic to neutral conditions (pH 5-7). Under acidic conditions (pH < 4), competition between metal ions and protons for amino groups reduces uptake, while at higher pH values, metal hydrolysis and precipitation may occur [41].
Regeneration studies indicate that cross-linked composites maintain their adsorption capacity over multiple cycles (4-7 cycles) due to enhanced mechanical and chemical stability [41]. Dilute acid solutions (HCl or HNO₃, 0.1-0.5 M) effectively desorb bound metals without significantly degrading the chitosan matrix.
The Scientist's Toolkit: Essential Research Reagents
Table 3: Essential Reagents for Nanomagnetic Chitosan Composite Synthesis
| Reagent | Function | Typical Concentration/Purity | Handling Considerations |
|---|---|---|---|
| Chitosan | Polymer matrix providing adsorption sites | Low to medium molecular weight; >75% deacetylation | Soluble in dilute acetic acid; avoid bacterial contamination |
| FeCl₃·6H₂O / Fe(NO₃)₃·9H₂O | Iron precursor for magnetic nanoparticles | ≥98% purity; analytical grade | Moisture-sensitive; store in desiccator |
| NiCl₂·6H₂O / Co(NO₃)₂·6H₂O | Transition metal precursors for ferrites | ≥98% purity; analytical grade | Handle with appropriate PPE due to toxicity |
| NaOH | Precipitation agent and pH adjustment | 0.1-5 M solutions; pellet form | Highly exothermic when dissolved; use caution |
| Glutaraldehyde | Cross-linking agent | 1-2% v/v aqueous solution | Toxic; use in fume hood with proper ventilation |
| Acetic Acid | Solvent for chitosan | 1-2% v/v aqueous solution | Corrosive; use in well-ventilated area |
| Sodium Tripolyphosphate (TPP) | Ionic cross-linker for microsphere formation | 1-2% w/v aqueous solution | Hygroscopic; store in airtight container |
| Oleic Acid | Surfactant for particle size control | 0.01-0.1% v/v | Can form stable foams during mixing |
| Ethylene Glycol | Solvent medium for hydrothermal synthesis | 0-100% v/v with water | Reflux conditions required for some syntheses |
The selection of an appropriate synthesis method for nanomagnetic chitosan composites represents a critical decision point that fundamentally determines their efficacy in heavy metal removal applications. Co-precipitation offers simplicity and scalability, making it suitable for large-scale wastewater treatment operations. Cross-linking provides enhanced chemical stability and excellent adsorption capacities, particularly beneficial for treating complex wastewater streams with variable pH conditions. Hydrothermal synthesis enables precise morphological control and high crystallinity, advantageous for fundamental studies and applications requiring specific structural features.
Future research should focus on optimizing synthesis parameters to enhance adsorption selectivity for specific heavy metals, improving regeneration efficiency for long-term use, and developing hybrid approaches that combine the advantages of multiple methods. The integration of characterization techniques with computational modeling will further advance our understanding of structure-property relationships, facilitating the rational design of next-generation nanocomposite adsorbents for environmental remediation.
In the pursuit of effective heavy metal remediation, chitosan has emerged as a promising biodegradable adsorbent. However, its inherent limitations, including poor mechanical strength, pH sensitivity, and difficult post-adsorption separation, restrict its practical application [47] [48]. To overcome these challenges, strategic modifications are employed. The integration of a magnetic core facilitates easy separation using an external magnet, significantly improving practicality [47] [49]. Further functionalization through crosslinking or composite formation enhances stability and adsorption capacity.
This guide focuses on three strategic modifications: crosslinking with Tripolyphosphate (TPP), crosslinking with Vanillin, and compositing with Cellulose. It objectively compares their performance in removing heavy metals, supported by experimental data and detailed methodologies, providing a clear framework for selecting and implementing these modifications in water treatment research.
Comparative Analysis of Modification Strategies
The performance of modified magnetic chitosan composites varies significantly based on the functionalization strategy and target heavy metal. The table below summarizes key performance metrics from recent research.
Table 1: Comparative Adsorption Performance of Functionalized Magnetic Chitosan Composites
| Functionalization Strategy | Target Heavy Metal | Reported Adsorption Capacity (mg/g) | Key Experimental Conditions | Primary Adsorption Mechanism(s) |
|---|---|---|---|---|
| TPP Crosslinking | Various (Cu, Pb, Cr) | Varies by specific composite design [47] | pH, temperature, and initial concentration dependent [47] | Ionic interaction, Electrostatic attraction [47] |
| Vanillin Crosslinking | (Mainly improves mechanical properties) [50] | (Not the primary focus of study) [50] | --- | Covalent crosslinking (improves matrix stability) [50] [51] |
| Cellulose Composite (Aerogel) | Cu(II) | 200.6 | Not specified | Coordination, Chelation [52] |
| Cellulose Composite (Aerogel) | Cr(VI) | 152.1 | Not specified | Coordination, Chelation [52] |
Detailed Experimental Protocols
Synthesis of Magnetic Chitosan (Base Material)
The coprecipitation method is a common and efficient technique for synthesizing the magnetic chitosan base material. The following workflow outlines the key steps for preparing crosslinked magnetic chitosan composites, starting with this base synthesis.
Diagram Title: Composite Preparation Workflow
In Situ Coprecipitation Method
This one-pot method is widely used for its simplicity [47].
- Step 1: Chitosan Dissolution. Chitosan is dissolved in a dilute acetic acid solution (e.g., 1-2% v/v) to obtain a homogeneous polymer solution [47].
- Step 2: Iron Ion Incorporation. Ferric (Fe³⁺) and ferrous (Fe²⁺) chloride solutions are added to the chitosan solution under an inert nitrogen atmosphere to prevent oxidation. The mixture is vigorously stirred to ensure uniform dispersion of ions within the polymer matrix [47].
- Step 3: Precipitation & Magnetic Formation. The solution is heated to 50-60°C, and an ammonium hydroxide solution is added dropwise under continuous stirring to raise the pH to 9-10. This alkaline environment causes the simultaneous precipitation of magnetite (Fe₃O₄) nanoparticles and their integration with the chitosan chains [47].
- Step 4: Separation and Washing. The resulting magnetic chitosan (MCS) particles are separated from the suspension using an external magnet, washed repeatedly with deionized water and ethanol until neutral pH is achieved, and then dried [47].
Functionalization with Tripolyphosphate (TPP)
TPP is an anionic crosslinker that forms bridges with the protonated amino groups of chitosan through strong ionic interactions [47] [51].
- Crosslinking Procedure: The synthesized magnetic chitosan particles are dispersed in a deionized water and agitated. Aqueous Sodium Tripolyphosphate (TPP) solution is added dropwise into this dispersion. The crosslinking reaction proceeds for a specified duration (e.g., 2-24 hours) with constant stirring [51]. The TPP-crosslinked magnetic chitosan (MCS-TPP) is then separated magnetically, washed, and dried.
Functionalization with Vanillin
Vanillin acts as a covalent crosslinker for chitosan, forming Schiff base linkages between its aldehyde groups and the primary amine groups of chitosan. This reaction significantly improves the mechanical properties of the chitosan matrix [50] [51].
- Crosslinking Procedure: The magnetic chitosan base is immersed in a 5% (w/v) vanillin solution prepared in ethanol. The mixture is agitated for a set period, such as 24 hours, to allow the crosslinking reaction to complete [51]. The resulting vanillin-crosslinked magnetic chitosan (MCS-Vanillin) is separated using a magnet, thoroughly washed with ethanol and water to remove unreacted vanillin, and dried.
Compositing with Cellulose
Incorporating cellulose, particularly in the form of bacterial cellulose nanofibers or as carboxymethyl cellulose, can enhance the porosity, water stability, and specific surface area of the composite adsorbent [52] [53] [54].
- Aerogel Composite Procedure: Chitosan and cellulose (or its derivatives) are co-dissolved in a suitable mutual solvent or blended in solution. Magnetic particles can be incorporated during this mixing step. The homogeneous mixture is then poured into a mold and subjected to a freeze-drying (lyophilization) process to create a porous three-dimensional aerogel structure [52] [53]. In some protocols, the composite is further crosslinked with agents like genipin or TPP after formation to improve its stability in aqueous environments [53].
The Scientist's Toolkit: Essential Research Reagents
The table below lists key materials required for the synthesis and evaluation of functionalized magnetic chitosan composites.
Table 2: Essential Reagents for Composite Synthesis and Testing
| Reagent/Material | Function in Research | Key Characteristics & Considerations |
|---|---|---|
| Chitosan | Primary adsorbent matrix; provides amino and hydroxyl groups for metal binding and crosslinking. | Degree of deacetylation (affects amine group density) and molecular weight are critical parameters [48]. |
| FeCl₂·4H₂O & FeCl₃·6H₂O | Iron precursors for the in-situ synthesis of magnetite (Fe₃O₄) nanoparticles. | Molar ratio of Fe²⁺/Fe³⁺ is typically maintained at 1:2 for stoichiometric magnetite formation [47]. |
| Sodium Tripolyphosphate (TPP) | Ionic crosslinker; enhances chemical stability and mechanical strength. | Concentration and reaction time influence crosslinking density and porosity [47] [51]. |
| Vanillin | Covalent crosslinker; significantly improves mechanical properties of the chitosan film/composite. | The aldehyde group forms Schiff bases with chitosan amines; often requires ethanol as a solvent [50] [51]. |
| Cellulose (Bacterial or CMC) | Biopolymer for composite formation; improves structural integrity, porosity, and specific surface area. | Bacterial cellulose offers high purity and nanofibril structure; Carboxymethyl Cellulose (CMC) introduces additional carboxyl groups for metal binding [52] [53] [55]. |
| Ammonium Hydroxide (NH₄OH) | Precipitating agent; creates alkaline conditions necessary for magnetite formation during coprecipitation. | Concentration and addition rate affect the size and crystallinity of magnetic nanoparticles [47]. |
| Acetic Acid | Solvent for dissolving chitosan by protonating amine groups. | Concentration should be optimized to fully dissolve chitosan without causing excessive degradation. |
The strategic functionalization of magnetic chitosan with TPP, Vanillin, or Cellulose significantly enhances its suitability for heavy metal removal applications. TPP crosslinking is a straightforward method that improves stability and leverages ionic interactions for metal binding. Vanillin crosslinking is highly effective for applications where superior mechanical strength is a priority. Conversely, compositing with cellulose to form aerogels creates a high-surface-area, porous network, demonstrating exceptional adsorption capacities for metals like copper and chromium.
The choice of modification strategy depends on the target heavy metal, desired material properties, and operational requirements. This comparative guide provides a foundation for researchers to select and optimize the most appropriate functionalization method for their specific environmental remediation goals.
The pervasive issue of heavy metal contamination in water bodies necessitates the development of efficient, sustainable, and cost-effective remediation technologies. Among various approaches, adsorption is widely favored for its simplicity and high efficiency [47]. In recent decades, magnetic chitosan composites have emerged as a premier class of adsorbents, synergizing the exceptional metal-binding capacity of chitosan—a biodegradable polysaccharide derived from chitin—with the facile separation capability of magnetic particles [47]. To enhance performance and economic viability, researchers have engineered sophisticated composite formulations by incorporating metal oxides, secondary polymers, and waste materials such as fly ash.
This guide provides an objective comparison of these advanced composite formulations, focusing on their performance in removing heavy metals from aqueous solutions. It is structured within a broader thesis on the comparative removal efficiency of different nanomagnetic chitosan composites, presenting synthesized experimental data, detailed methodologies, and analytical tools for researchers and scientists in environmental technology and drug development.
Performance Comparison of Composite Formulations
The removal efficiency of magnetic chitosan composites is highly influenced by their specific constituents. The table below provides a quantitative comparison of different composite formulations for removing specific heavy metals, based on recent experimental studies.
Table 1: Performance Comparison of Magnetic Chitosan Composite Formulations
| Composite Formulation | Target Heavy Metal | Optimal pH | Maximum Adsorption Capacity (mg/g) | Removal Efficiency (%) | Key Removal Mechanism(s) | Reference |
|---|---|---|---|---|---|---|
| PCC/Fe₃O₄ (Phosphorus-modified) | Cr(VI) | 6 | 23.6 mg/g | >90% (maintained over 5 cycles) | Electrostatic attraction, Redox reaction, Ligand exchange | [56] |
| Magnetic Chitosan/Sludge Biochar | Cu(II) | 5 | 55.16 mg/g | 99.77% | Chemisorption, Monolayer coverage | [57] |
| Chitosan-Al₂O₃ Hydrogel | Cr(VI) | 2 | ~50.2 mg/g (average) | High (stable over 4 cycles) | Multilayer adsorption, Complexation | [58] |
| Chitosan-Fly Ash/Fe₃O₄ | Reactive Orange 16 Dye | 4 | 66.9 mg/g | 73.1% | Electrostatic interaction, H-bonding, Yoshida H-bonding | [59] |
Analysis of Comparative Performance
- Synergy with Waste Materials: Composites incorporating waste-based materials like sludge biochar and fly ash demonstrate significantly enhanced adsorption capacities [57] [59]. This is attributed to the increased surface area and porosity provided by the waste matrix, which offers more active sites for metal binding. The use of waste materials also aligns with circular economy principles, reducing overall adsorbent cost.
- Role of Metal Oxides: The incorporation of Al₂O₃ (aluminum oxide) into chitosan hydrogels creates a robust inorganic core that enhances the mechanical strength and stability of the composite during repeated sorption/desorption cycles [58]. This reinforcement allows the hydrogel to maintain its structural integrity, thereby improving its reusability.
- Impact of Cross-linking and Functionalization: The phosphorus-modified magnetic chitosan (PCC/Fe₃O₄) exhibits excellent stability and reusability due to the cross-linking action of tetrakis hydroxymethyl phosphonium sulfate (THPS) [56]. This chemical modification prevents the dissolution of chitosan in acidic environments and enhances its affinity for target metals through specific functional groups.
Detailed Experimental Protocols
To ensure reproducibility and provide a clear basis for comparison, this section outlines the standardized methodologies employed in the synthesis and evaluation of the featured composites.
Composite Synthesis Protocols
Protocol A: Synthesis of Phosphorus-Modified Magnetic Chitosan (PCC/Fe₃O₄) This two-step method involves coating pre-synthesized magnetite with chitosan followed by cross-linking [56].
- Dispersion: Dissolve 2.0 g of chitosan in 200 mL of aqueous acetic acid (0.5% v/v) by magnetic stirring for 2 hours.
- Coating: Under a nitrogen atmosphere, add 1.0 g of pre-formed Fe₃O₄ nanoparticles (<50 nm) to the chitosan solution. Ultrasonicate for 1 hour to achieve a homogeneous dispersion.
- Cross-linking: Add 20 mL of tetrakis(hydroxymethyl)phosphonium sulfate (THPS, 75% solution) to the mixture.
- Reaction: Heat the reaction mixture to 70°C in a water bath with continuous stirring (320 rpm) for 1 hour.
- Recovery and Drying: Isolate the solid product via centrifugation at 3500 rpm for 10 minutes. Wash sequentially with deoxygenated water and ethanol. Freeze-dry the final product at -45°C for 48 hours.
Protocol B: Preparation of Chitosan-Al₂O₃ Green Hydrogel Composites This protocol uses gamma irradiation, a facile and eco-friendly technique, to induce cross-linking [58].
- Solution Preparation: Dissolve specified quantities of chitosan (or carboxymethyl chitosan) in 100 mL of a 5% acetic acid solution. Sonicate for 30 minutes to achieve homogeneity.
- Mixing: Add a dissolved quantity of acrylamide (AAm) and 0.05 g of Al₂O₃ to the chitosan solution. Stir continuously for two hours at 50–60°C.
- Cross-linking: Transfer the final composition into Pyrex glass tubes and expose to a gamma irradiation dose of 30 kGy to form hydrogel rods.
- Post-processing: Slice the hydrogel rods into uniform discs, air-dry, and purify by extraction in distilled water at 70°C for 2 hours.
Batch Adsorption Experiment Protocol
The following is a standard batch adsorption procedure used to generate the performance data in Table 1 [56] [57].
- Solution Preparation: Prepare a stock solution of the target heavy metal (e.g., K₂Cr₂O₇ for Cr(VI)) at a known concentration using deionized water.
- pH Adjustment: Adjust the pH of the metal solution to the desired value using dilute NaOH or HCl solutions (e.g., 0.1 M).
- Adsorption Process: Mix a precise dosage of the composite adsorbent with a fixed volume (e.g., 250 mL) of the metal solution in a flask.
- Agitation: Agitate the mixture at a constant speed (e.g., 180 rpm) and temperature (e.g., 25°C) for a predetermined contact time.
- Separation: After agitation, separate the adsorbent from the solution using magnetic separation or filtration (e.g., through a 0.45 μm membrane).
- Analysis: Quantify the residual metal concentration in the filtrate using an appropriate analytical technique, such as inductively coupled plasma-atomic emission spectrometry (ICP) or UV-Vis spectrophotometry (e.g., via the diphenylcarbazide method for Cr(VI) at 540 nm).
The adsorption capacity (qₑ, mg/g) and removal efficiency are calculated using the following formulas, where C₀ and Cₑ are the initial and equilibrium concentrations (mg/L), V is the volume of solution (L), and m is the mass of adsorbent (g) [56]:
- Adsorption Capacity: ( qe = V \times (C0 - C_e) / m )
- Removal Efficiency: ( \text{Removal efficiency} = (C0 - Ce)/C_0 \times 100\% )
Visualization of Workflow and Mechanisms
The following diagram illustrates the logical workflow from composite synthesis to performance evaluation, integrating the key components and processes described in the experimental protocols.
Figure 1: A logical workflow for developing and evaluating magnetic chitosan composites, from initial synthesis to final performance assessment.
The removal of heavy metals by magnetic chitosan composites involves multiple mechanisms, often acting in concert. The diagram below outlines the primary mechanisms through which these composites interact with and retain heavy metal ions.
Figure 2: Key mechanisms involved in the adsorption of heavy metals onto magnetic chitosan composites.
The Scientist's Toolkit: Essential Research Reagents and Materials
The development and testing of magnetic chitosan composites require a set of essential reagents and materials. The table below details key items and their specific functions in the synthesis and evaluation processes.
Table 2: Essential Research Reagents and Materials for Composite Development
| Reagent/Material | Function/Application | Key Characteristics & Considerations |
|---|---|---|
| Chitosan | Primary biopolymer matrix; provides adsorption sites | Source (e.g., crustacean shells), degree of deacetylation (≥95%), molecular weight [56] [47]. |
| Fe₃O₄ (Magnetite) | Magnetic core for facile separation | Particle size (<50 nm), purity (>99%), synthesis method (e.g., co-precipitation) [56] [47]. |
| Tetrakis Hydroxymethyl Phosphonium Sulfate (THPS) | Cross-linking agent for chitosan | Enhances chemical stability and reusability; introduces phosphorus functional groups [56]. |
| Sludge Biochar / Fly Ash | Waste-derived substrate to enhance surface area and porosity | Inexpensive, readily available; improves adsorption capacity and reduces material cost [57] [59]. |
| Aluminum Oxide (Al₂O₃) | Inorganic filler to reinforce hydrogel structure | Improves mechanical strength, thermal stability, and reusability of the composite [58]. |
| Acetic Acid | Solvent for dissolving chitosan | Concentration (e.g., 0.5-5% v/v) is critical for creating a homogeneous chitosan solution [56] [58]. |
| Gamma Irradiation | Environmentally friendly method for cross-linking hydrogels | Ensures uniform cross-linking without toxic chemical initiators; optimized dose (e.g., 30 kGy) [58]. |
This comparison guide demonstrates that the strategic formulation of magnetic chitosan composites directly dictates their adsorption performance and practical applicability. Composites enhanced with waste materials (fly ash, sludge biochar) offer a compelling balance of high efficiency and low cost, making them suitable for large-scale applications. Those reinforced with metal oxides (e.g., Al₂O₃) or specialized cross-linkers (e.g., THPS) excel in scenarios demanding exceptional stability and reusability. The choice of optimal composite depends on the specific target metal, operational conditions (especially pH), and the overarching project goals, which may prioritize maximum capacity, cost-effectiveness, or long-term durability. This systematic comparison provides a foundational framework for researchers to select and further innovate upon these promising adsorbents.
In the field of wastewater remediation, the removal of toxic heavy metals is a critical environmental challenge. Among various remediation technologies, adsorption is widely regarded as a superior method due to its high efficiency, operational simplicity, and cost-effectiveness [60]. The search for advanced adsorbents has led to the development of engineered materials, with nanomagnetic chitosan composites emerging as a particularly promising class of adsorbents that combine the excellent metal-binding capacity of chitosan with the magnetic separation capability of iron oxides [5] [6].
This guide provides a systematic comparison of the adsorption performance, measured by maximum adsorption capacity (mg/g), for various adsorbents targeting four heavy metals of significant environmental concern: lead (Pb), chromium (Cr), copper (Cu), and cadmium (Cd). The data presented serves as a reference for researchers and engineers selecting appropriate materials for specific heavy metal remediation applications.
Comparative Adsorption Performance Data
The adsorption capacity of an adsorbent is predominantly quantified by the maximum amount of heavy metal ions adsorbed per unit mass of adsorbent (mg/g), typically derived from Langmuir isotherm models. The table below summarizes experimental capacity data for various adsorbent categories.
Table 1: Comparative adsorption capacity (mg/g) of various adsorbents for heavy metals
| Adsorbent Category | Specific Material | Lead (Pb) | Chromium (Cr) | Copper (Cu) | Cadmium (Cd) | Citation |
|---|---|---|---|---|---|---|
| Magnetic Chitosan Composites | Magnetic Chitosan Composite (MCC) | 220.9 mg/g | Not Reported | 216.8 mg/g | Not Reported | [5] [6] |
| Modified Natural Adsorbents | ZnO-Modified Date Pits (MDP) | Not Reported | Not Reported | 82.4 mg/g | Not Reported | [61] |
| Not Reported | Not Reported | Not Reported | 71.9 mg/g (Ni(II))* | [61] | ||
| Agricultural Waste-Based | Peanut Shells (PS) | High Affinity | Not Reported | Medium Affinity | Medium Affinity | [62] |
| Sawdust (S) | High Affinity | Not Reported | Medium Affinity | Medium Affinity | [62] | |
| Commercial Benchmark | Activated Carbon (AC) | High Affinity | Not Reported | Medium Affinity | Medium Affinity | [62] |
Note: The study on ZnO-Modified Date Pits focused on Cu(II), Ni(II), and Zn(II). Data for Ni(II) is included for reference due to its chemical similarity to Cd(II), though they are different metals.
Performance Analysis
- Lead Removal: Magnetic Chitosan Composite (MCC) demonstrates exceptional affinity for lead, with a capacity of 220.9 mg/g [5] [6]. Comparative studies on low-cost adsorbents also consistently identify lead as the metal with the highest removal rates across different materials [62].
- Copper Removal: MCC also shows a very high capacity for copper (216.8 mg/g) [5] [6]. Modified natural materials like ZnO-Modified Date Pits achieve a respectable 82.4 mg/g for copper, highlighting the performance enhancement possible through material modification [61].
- Material Versatility: Magnetic chitosan composites are noted for their promising versatility in treating wastewater containing multiple pollutants, showing high capacity for both heavy metals and organic compounds [5].
Experimental Protocols for Adsorption Studies
The experimental data cited in this guide were generated through standardized batch adsorption studies. The following section outlines the core methodologies employed.
Batch Adsorption Experiments
This is the most common approach for evaluating adsorption capacity and kinetics [62] [61]. The general workflow involves preparing metal solutions, mixing with the adsorbent under controlled conditions, and analyzing the residual metal concentration.
Diagram 1: Batch adsorption experiment workflow for determining adsorption capacity.
Key Protocol Steps:
- Solution Preparation: Mother liquors of heavy metal ions (e.g., Pb(II), Cu(II), Cd(II)) are prepared by dissolving specific salts like Pb(NO₃)₂, Cu(NO₃)₂·2H₂O, or Cd(NO₃)₂·4H₂O in distilled water, typically at a stock concentration of 1000 mg/L [62].
- pH Adjustment: The pH of the solution is adjusted using acids (HNO₃) or bases (NaOH) to the desired value, as pH significantly impacts metal speciation and adsorbent surface charge [62] [61].
- Adsorbent Addition: A precise mass of the adsorbent (e.g., 0.4 g) is added to a known volume of the metal solution (e.g., 35 mL) [62].
- Agitation: The mixture is agitated in a shaker at a constant speed (e.g., 150-400 rpm) and temperature for a predetermined contact time to reach equilibrium [61].
- Separation: The adsorbent is separated from the liquid phase. Magnetic composites like MCC are easily separated using an external magnet [5], while other adsorbents require centrifugation or filtration [62].
- Analysis: The equilibrium concentration of the heavy metal in the supernatant (Cₑ) is analyzed using techniques like Inductively Coupled Plasma (ICP) or Atomic Absorption Spectroscopy (AAS) [62].
- Calculation: The adsorption capacity at equilibrium (qₑ, mg/g) is calculated using the formula: qₑ = (C₀ - Cₑ) × V / M, where C₀ is the initial concentration (mg/L), V is the solution volume (L), and M is the mass of the adsorbent (g) [62].
Adsorption Isotherm and Kinetic Modeling
To understand the adsorption process and quantify capacity, experimental data is fitted to mathematical models.
Table 2: Key models for analyzing adsorption data
| Model Type | Model Name | Primary Application | Key Parameters |
|---|---|---|---|
| Isotherm | Langmuir | Models monolayer adsorption on a homogeneous surface; estimates maximum capacity. | qₘ (max capacity), b (affinity constant) |
| Freundlich | Models adsorption on a heterogeneous surface. | K_F (capacity constant), n (intensity) | |
| Kinetic | Pseudo-First-Order | Analyzes adsorption kinetics based on adsorbate concentration. | k₁ (rate constant), qₑ (equilibrium capacity) |
| Pseudo-Second-Order | Assumes chemisorption is the rate-limiting step. | k₂ (rate constant), qₑ (equilibrium capacity) |
Application in Cited Studies:
- The high capacities for MCC and MDP were derived from the Langmuir isotherm model, indicating monolayer adsorption [5] [61].
- The adsorption kinetics for MCC reached equilibrium within 120 minutes and were well-described by pseudo-second-order kinetics, suggesting a chemisorption process [5] [6].
The Scientist's Toolkit: Essential Research Reagents and Materials
This table details key reagents, materials, and instruments essential for conducting adsorption experiments as described in the cited research.
Table 3: Essential research reagents and materials for adsorption studies
| Item Name | Specification / Example | Primary Function in Experiment |
|---|---|---|
| Heavy Metal Salts | Pb(NO₃)₂, Cu(NO₃)₂·2H₂O, Cd(NO₃)₂·4H₂O | Source of heavy metal ions (adsorbate) for preparing stock and test solutions. |
| pH Modifiers | NaOH, HNO₃ (Analytical Grade) | To adjust the pH of the solution, a critical parameter controlling adsorption efficiency. |
| Chitosan | Commercial Chitosan Flakes | Biopolymer base for synthesizing chitosan-based composite adsorbents. |
| Magnetic Precursor | Fe₃O₄ / FeCl₂/FeCl₃ | Provides magnetic properties to composites for easy separation post-adsorption. |
| Natural Adsorbents | Peanut Shells, Sawdust, Date Pits | Raw, low-cost materials used as-is or as a base for modified adsorbents. |
| Modifying Agents | ZnO, ZnCl₂, KOH | Chemicals used to activate or functionalize raw adsorbents to enhance surface area and reactivity. |
| Analytical Instrument | Inductively Coupled Plasma (ICP) or AAS | Precisely measures heavy metal concentration in solution before and after adsorption. |
| FT-IR Spectrometer | PerkinElmer Spectrum 2000 | Characterizes functional groups on the adsorbent surface involved in metal binding. |
| Surface Area Analyzer | BET Method (e.g., Thermo Scientific Surfer) | Determines the specific surface area and pore structure of the adsorbent. |
This comparison guide objectively presents the adsorption performance of various materials for key heavy metals. The data unequivocally shows that nanomagnetic chitosan composites can achieve superior adsorption capacities, particularly for lead (220.9 mg/g) and copper (216.8 mg/g), outperforming many modified natural and agricultural waste-based adsorbents [5] [6]. This, combined with their magnetic separability, positions them as a versatile and highly effective option for wastewater remediation.
The choice of adsorbent, however, must also consider factors like cost, specificity for a particular metal, and operational conditions. The experimental protocols and toolkit outlined provide a foundation for researchers to consistently evaluate and develop next-generation adsorbents for environmental applications.
The removal of heavy metals from wastewater using nanomagnetic chitosan composites (NMCCs) has emerged as a prominent field of research in environmental remediation. While numerous studies have synthesized and applied these adsorbents, a critical understanding of the underlying adsorption mechanisms is essential for optimizing their design and application. This is where the interpretation of kinetic and isotherm models becomes indispensable. Among the various models, the pseudo-second-order (PSO) kinetic and Langmuir isotherm models are most frequently employed to provide fundamental insight into the adsorption process. This guide objectively compares the performance of different NMCCs by examining how these models are used to elucidate adsorption mechanisms, supported by experimental data from key studies in the field. The analysis is framed within the broader thesis of evaluating the comparative removal efficiency of different NMCCs for heavy metals, providing researchers with a standardized framework for mechanistic interpretation.
Theoretical Framework: Decoding the Models
Pseudo-Second-Order Kinetic Model
The PSO model is a cornerstone in adsorption studies for analyzing the rate of metal uptake and the potential mechanisms at play. The model's linearized form is expressed as: [ t/qt = 1/(k2 qe^2) + t/qe ] where ( qe ) and ( qt ) are the amounts of metal adsorbed (mg g⁻¹) at equilibrium and at time ( t ), respectively, and ( k_2 ) is the PSO rate constant (g mg⁻¹ min⁻¹) [63] [64].
When experimental data exhibits a strong fit to the PSO model (typically indicated by a high regression coefficient, R², close to 1), it suggests that the rate-limiting step of the adsorption process is chemisorption [65] [64]. This involves the sharing or exchange of electrons between the adsorbent (NMCC) and the adsorbate (metal ion), often through strong coordinate covalent bonds with the amino (–NH₂) and hydroxyl (–OH) functional groups on the chitosan backbone [65] [47]. The model's parameters, ( qe ) and ( k2 ), allow for quantitative comparisons of the maximum adsorption capacity and the adsorption rate between different NMCCs under defined conditions.
Langmuir Isotherm Model
The Langmuir isotherm model describes adsorption on a homogeneous surface where monolayer coverage is assumed, with no transmigration of adsorbate in the surface plane. Its linear form is given by: [ Ce/qe = 1/(KL qm) + Ce/qm ] where ( Ce ) is the equilibrium concentration of the metal ion (mg L⁻¹), ( qm ) is the maximum monolayer adsorption capacity (mg g⁻¹), and ( K_L ) is the Langmuir constant (L mg⁻¹) related to the affinity of binding sites [63] [64].
A successful application of the Langmuir model implies that the surface of the NMCC is homogeneous in energy, and each metal ion has equal affinity for the adsorption sites, with no interaction between adsorbed ions [63] [66]. The model's parameters are crucial for cross-comparing different composites. The ( qm ) value provides a theoretical maximum capacity, enabling a direct performance comparison. Furthermore, the essential characteristic of the Langmuir isotherm can be described by a dimensionless separation factor, ( RL ), calculated as ( RL = 1 / (1 + KL C0) ), where ( C0 ) is the initial metal concentration. An ( R_L ) value between 0 and 1 indicates favorable adsorption [64].
Table 1: Key Parameters from Kinetic and Isotherm Models and Their Interpretative Significance
| Model | Key Parameter | Symbol | Unit | Interpretative Significance |
|---|---|---|---|---|
| Pseudo-Second-Order Kinetics | Equilibrium Adsorption Capacity | ( q_e ) | mg g⁻¹ | Theoretical amount of metal adsorbed per gram of adsorbent at equilibrium. |
| Rate Constant | ( k_2 ) | g mg⁻¹ min⁻¹ | Indicates the speed of the adsorption process; a higher value suggests faster uptake. | |
| Langmuir Isotherm | Maximum Monolayer Capacity | ( q_m ) | mg g⁻¹ | Theoretical maximum adsorption capacity assuming a homogeneous surface. |
| Langmuir Constant | ( K_L ) | L mg⁻¹ | Reflects the affinity between the adsorbent and the metal ions; a higher value indicates stronger affinity. | |
| Separation Factor | ( R_L ) | Dimensionless | Predicts adsorption favorability: ( RL > 1 ) (unfavorable), ( RL = 1 ) (linear), ( 0 < RL < 1 ) (favorable), ( RL = 0 ) (irreversible). |
Comparative Performance of Nanomagnetic Chitosan Composites
Experimental data from recent studies consistently demonstrate that modified NMCCs exhibit high removal efficiencies for various heavy metals, with their adsorption processes being well-described by the PSO and Langmuir models. The following table provides a comparative summary of the performance of different composites.
Table 2: Comparative Adsorption Performance of Various Nanomagnetic Chitosan Composites for Heavy Metals
| Adsorbent | Target Metal | Pseudo-Second-Order ( q_e ) (mg g⁻¹) | Langmuir ( q_m ) (mg g⁻¹) | Optimum pH | Equilibrium Time (min) | Ref. |
|---|---|---|---|---|---|---|
| TPP-CMN | Cd(II) | - | 91.75 | - | 15 | [13] |
| Co(II) | - | 93.00 | - | 15 | [13] | |
| Cu(II) | - | 87.25 | - | 15 | [13] | |
| Pb(II) | - | 99.96 | - | 15 | [13] | |
| V-CMN | Cd(II) | - | 92.50 | - | 30 | [13] |
| Co(II) | - | 94.00 | - | 30 | [13] | |
| Cu(II) | - | 88.75 | - | 30 | [13] | |
| Pb(II) | - | 99.89 | - | 30 | [13] | |
| 4‑aminobenzoic acid grafted CS/Epichlorohydrin | Various | - | - | - | - | [67] |
| CS/Fe₃O₄ (Magnetic CS) | Cu(II) | - | - | - | - | [47] |
| Pb(II) | - | - | - | - | [47] | |
| Cr(VI) | - | - | - | - | [47] |
Key Observations:
- High Capacity and Rapid Uptake: Composites like TPP-CMN and V-CMN show exceptionally high adsorption capacities ( ( q_m ) > 87 mg g⁻¹ for all metals tested) and reach equilibrium remarkably quickly (15-30 minutes). This rapid kinetics is a significant advantage for practical applications [13].
- Superiority of Lead Removal: Across multiple studies, Pb(II) consistently shows one of the highest adsorption capacities (e.g., 99.96 mg g⁻¹ on TPP-CMN). This can be attributed to the high affinity of lead ions for the amino and other functional groups on chitosan, and its large ionic size which facilitates stronger complexation [13] [64].
- Role of Modification: The high performance of these composites is not inherent to raw chitosan but is a direct result of strategic modifications. Grafting with specific molecules (e.g., 4-aminobenzoic acid) or cross-linking with agents like epichlorohydrin and TPP creates more stable and accessible binding sites, enhancing both capacity and kinetics [67] [64].
Essential Research Reagents and Materials
The synthesis, modification, and application of NMCCs involve a range of key reagents and materials. The following table details these essential components and their functions in the research process.
Table 3: Key Research Reagent Solutions and Materials for NMCC Studies
| Reagent/Material | Function/Application in Research | Key References |
|---|---|---|
| Chitosan | Primary biosorbent backbone; provides amino and hydroxyl functional groups for metal coordination. | [65] [47] [64] |
| Fe₃O₄ (Magnetite) Nanoparticles | Provides magnetic core for easy separation of the composite from solution using an external magnet. | [13] [47] |
| Sodium Tripolyphosphate (TPP) | A common cross-linking agent used to enhance the mechanical and chemical stability of chitosan in acidic media. | [13] [64] |
| Epichlorohydrin | A cross-linker used to form a stable network within the chitosan polymer, improving its resistance to dissolution. | [64] [67] |
| Glutaraldehyde | Another widely used cross-linking agent that reacts with amino groups on chitosan. | [64] |
| Vanillin | A grafting agent used to functionalize the chitosan surface, introducing additional functional groups for metal binding. | [13] |
| 4-Aminobenzoic Acid | A grafting agent modified onto chitosan to enhance its adsorption capacity and selectivity for heavy metal ions. | [67] |
Experimental Protocols: From Synthesis to Mechanism Elucidation
To ensure the reproducibility and reliability of adsorption studies, a standardized set of experimental protocols is followed. The workflow below visualizes the key stages in a typical NMCC study.
Synthesis of Nanomagnetic Chitosan Composites
The co-precipitation method is one of the most common techniques for synthesizing NMCCs. A typical protocol involves two main steps [47]:
- Synthesis of Fe₃O₄ Nanoparticles: Aqueous solutions of Fe³⁺ and Fe²⁺ salts (e.g., FeCl₃ and FeSO₄) are mixed in an inert atmosphere at a specific molar ratio (often 2:1). A base, such as NaOH or NH₄OH, is then added under vigorous stirring and heating (e.g., 50-80°C) to precipitate the magnetic nanoparticles. The resulting black precipitate of Fe₃O₄ is washed with water and ethanol and dried [13] [47].
- Coating with Chitosan: The prepared Fe₃O₄ nanoparticles are dispersed into a solution of chitosan dissolved in dilute acetic acid (e.g., 1-3% v/v). The mixture is sonicated and stirred for several hours to ensure a homogeneous coating. Cross-linkers like TPP or glutaraldehyde may be added during this step to stabilize the composite. The final product is separated magnetically, washed, and dried [13] [47].
Batch Adsorption Experiments
Batch experiments are the standard for evaluating adsorption performance and generating data for kinetic and isotherm modeling [66] [64]:
- Kinetic Studies: A fixed mass of the NMCC (e.g., 0.01-0.1 g) is added to a series of containers with a fixed volume (e.g., 100-200 mL) and known initial concentration of the metal ion solution. The containers are agitated at a constant speed and temperature. Samples are withdrawn at predetermined time intervals, the adsorbent is separated (often magnetically), and the residual metal concentration in the supernatant is analyzed, typically using Atomic Absorption Spectroscopy (AAS) or Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES) [66] [13].
- Isotherm Studies: The procedure is similar, but the initial metal concentration is varied across a wide range (e.g., 10-500 mg L⁻¹) while keeping other parameters like adsorbent dose, pH, temperature, and contact time (sufficient to reach equilibrium) constant [66] [13].
Data Modeling and Validation
- Kinetic Modeling: The experimental data from the kinetic study ((qt) vs. (t)) is fitted to the linear or non-linear forms of the PFO and PSO models. The model with a higher coefficient of determination (R²) and a (q{e,calc}) value closer to the experimental (q_{e,exp}) is considered the best fit. Non-linear regression methods are increasingly recommended as a more accurate way to obtain the kinetic parameters [63].
- Isotherm Modeling: The equilibrium data ((Ce) and (qe)) is fitted to isotherm models like Langmuir and Freundlich. The Langmuir model's applicability is validated by a high R² and the calculation of the (R_L) factor, which should be between 0 and 1 for favorable adsorption [66] [64].
Elucidating the Adsorption Mechanism
The consistent, strong fit of experimental data for NMCCs to both the PSO kinetic and Langmuir isotherm models paints a clear picture of the dominant adsorption mechanism. The diagram below synthesizes the experimental workflow and the mechanistic insights derived from it.
The convergence of evidence from kinetics, isotherms, and material characterization strongly indicates that the adsorption of heavy metals onto NMCCs is predominantly a chemisorption process involving the formation of monolayers on specific, homogeneous sites [63] [66] [64]. The primary interaction is the complexation of metal cations with the lone pair of electrons on the nitrogen atoms of the amino groups (-NH₂) and, to a lesser extent, the oxygen atoms of the hydroxyl groups (-OH) on the chitosan chain [65] [47] [64]. This coordination is often pH-dependent, as the protonation state of the amino group changes with solution acidity. Modifications like grafting introduce additional functional groups (e.g., carboxyl from vanillin or 4-aminobenzoic acid), which can further enhance this complexation and introduce other interactions like electrostatic attraction, leading to the superior performance observed in modified composites like TPP-CMN and V-CMN [13] [67].
Overcoming Real-World Hurdles: Stability, Selectivity, and Scalability
The effectiveness of nanomagnetic chitosan composites in removing heavy metals from wastewater is highly dependent on several critical operational parameters. These parameters—pH, adsorbent dosage, temperature, and contact time—directly influence the adsorption capacity, kinetics, and overall efficiency of the remediation process. Understanding and optimizing these factors is essential for researchers and scientists seeking to deploy these advanced materials in real-world applications, from industrial wastewater treatment to environmental remediation. This guide provides a comparative analysis of how these parameters affect the performance of various nanomagnetic chitosan composites, supported by experimental data from recent studies.
Operational Parameters: A Comparative Analysis
The performance of nanomagnetic chitosan composites is governed by a complex interplay of operational conditions. The tables below summarize key experimental findings from recent research, providing a direct comparison of how different parameters influence adsorption efficiency across various composite types and target metals.
Table 1: Comparison of Key Operational Parameters Across Different Composites
| Composite Type | Target Pollutant | Optimal pH | Optimal Dosage (g/L) | Equilibrium Time (min) | Temperature Effect | Reference |
|---|---|---|---|---|---|---|
| Cubebinol-Chitosan Beads | Cr(VI), Ni(II) | Specific pH critical [68] | Optimized via RSM [68] | Optimized via RSM [68] | --- | [68] |
| Chitosan-Lignin Biocomposite | Cr(VI) | 2 [29] | 2.0 [29] | --- | Adsorption exothermic [29] | [29] |
| TPP-CMN | Cd(II), Co(II), Cu(II), Pb(II) | --- | --- | 15 [13] | --- | [13] |
| V-CMN | Cd(II), Co(II), Cu(II), Pb(II) | --- | --- | 30 [13] | --- | [13] |
| EDTA-M-Cs | Cu(II), Cd(II), Zn(II) | Higher pH favored [69] | --- | --- | Process endothermic [69] | [69] |
| M-Ch/CNF-Fe(III) | Cr(VI), Cu(II), Pb(II) | 1.0-8.0 (varies by metal) [12] | 0.05-1.0 [12] | 15-90 [12] | 28-80°C (varies) [12] | [12] |
Table 2: Comparison of Adsorption Performance Metrics
| Composite Type | Max Adsorption Capacity (mg/g) | Adsorption Isotherm Model | Adsorption Kinetic Model | Key Removal Efficiency | Reference |
|---|---|---|---|---|---|
| Chitosan-Lignin Biocomposite | RO16 Dye: 59.43-79.76; Cr(VI): 52.06-72.61 [29] | Statistical Physics Model [29] | --- | --- | [29] |
| TPP-CMN | Pb(II): 99.96; Co(II): 93.00; Cd(II): 91.75; Cu(II): 87.25 [13] | --- | --- | --- | [13] |
| V-CMN | Pb(II): 99.89; Co(II): 94.00; Cd(II): 92.5; Cu(II): 88.75 [13] | --- | --- | --- | [13] |
| M-Ch/CNF-Fe(III) | --- | --- | --- | High for Cr(VI), Cu(II), Pb(II) [12] | [12] |
In-Depth Parameter Analysis
pH
The pH of the solution is arguably the most critical parameter as it governs the surface charge of the adsorbent, the degree of ionization, and the speciation of metal ions [29] [69].
- Chitosan-lignin biocomposite for Cr(VI) removal: Maximum adsorption occurs at pH 2. In highly acidic conditions, the chitosan's amino groups are protonated, fostering strong electrostatic attraction with the anionic chromate ions (Cr₂O₇²⁻) [29].
- EDTA-functionalized magnetic chitosan (EDTA-M-Cs): Removal of Cd(II), Zn(II), and Cu(II) increases with increasing pH. At lower pH, high H⁺ ion concentration competes with metal cations for adsorption sites [69].
Adsorbent Dosage
The adsorbent dosage determines the number of available binding sites. Optimization is crucial for cost-effectiveness.
- M-Ch/CNF-Fe(III) composite: Effective dosage for removing Cr(VI), Cu(II), and Pb(II) ranges from 0.05 to 1.0 g [12].
- Cubebinol-chitosan beads: The optimal dosage was systematically determined using Response Surface Methodology (RSM), highlighting the value of statistical optimization for such parameters [68].
Contact Time and Kinetics
Contact time determines the process duration required to reach equilibrium, which is vital for designing treatment systems.
- TPP-CMN and V-CMN nano-sorbents exhibit remarkably fast uptake, reaching equilibrium within 15 and 30 minutes, respectively, for Cd(II), Co(II), Cu(II), and Pb(II) ions. This rapid kinetics is a significant advantage for continuous flow applications [13].
- M-Ch/CNF-Fe(III) composite: Equilibrium times for metal removal were found to be between 15 and 90 minutes [12].
- The adsorption kinetics for most of these composites typically follow the pseudo-second-order model, indicating that chemisorption is the rate-limiting step [13] [14].
Temperature
Temperature influences the adsorption thermodynamics and the stability of the composite.
- Chitosan-lignin biocomposite: The adsorption of RO16 dye and Cr(VI) is an exothermic process, meaning adsorption capacity decreases with increasing temperature [29].
- EDTA-M-Cs composite: In contrast, the adsorption of Cu(II), Cd(II), and Zn(II) is endothermic, with capacity increasing as temperature rises. Thermodynamic studies confirmed the spontaneity of this process [69].
Experimental Protocols for Parameter Optimization
A standardized approach is key for obtaining comparable and reliable data on composite performance.
Protocol 1: Batch Adsorption Experiment for Isotherm and Kinetics
This is a fundamental method for evaluating adsorbent capacity and speed [29] [13].
- Stock Solution Preparation: Dissolve heavy metal salts (e.g., K₂Cr₂O₇ for Cr(VI), Pb(NO₃)₂ for Pb(II)) in deionized water to prepare a stock solution (e.g., 500-1000 mg/L).
- Experimental Setup: Prepare a series of containers (e.g., Erlenmeyer flasks) with fixed volumes (e.g., 50 mL) of metal solutions at varying initial concentrations (for isotherms) or a fixed concentration (for kinetics).
- pH Adjustment: Adjust the pH of each solution to the desired value using 0.1 M NaOH or HCl.
- Adsorption Initiation: Add a predetermined, precise mass of the nanomagnetic chitosan composite to each flask.
- Agitation and Sampling: Agitate the flasks at a constant speed (e.g., 240 rpm) and temperature. For kinetics, sample at regular time intervals (e.g., 5, 15, 30, 60, 90 min). For isotherms, sample once equilibrium is reached.
- Separation and Analysis: Separate the adsorbent using filtration or an external magnet. Analyze the supernatant for residual metal concentration using Atomic Absorption Spectroscopy (AAS) or UV-Vis spectrophotometry.
Protocol 2: Optimization Using Response Surface Methodology (RSM)
RSM is a powerful statistical technique for optimizing multiple parameters simultaneously [68].
- Factor Selection: Identify critical independent variables (e.g., pH, adsorbent dosage, contact time).
- Experimental Design: Utilize a design like the Box-Behnken Design (BBD) to create a set of experimental runs.
- Model Fitting: Perform the experiments according to the design and measure the response (e.g., removal percentage). Fit the data to a quadratic model.
- Analysis and Validation: Analyze the model to identify optimal parameter values and interaction effects between variables. Validate the model with confirmatory experiments.
Visualizing the Optimization Workflow
The following diagram illustrates the systematic process for optimizing the critical operational parameters of nanomagnetic chitosan composites.
The Scientist's Toolkit: Essential Research Reagents and Materials
This table details key materials and reagents commonly used in the synthesis and evaluation of nanomagnetic chitosan composites for heavy metal removal.
Table 3: Essential Research Reagents and Their Functions
| Reagent/Material | Function in Research | Example Application |
|---|---|---|
| Chitosan | Primary biopolymer backbone; provides amino & hydroxyl functional groups for metal binding [13] [12]. | Base material for all composites. |
| Fe₃O₄ (Magnetite) | Magnetic component; enables easy separation via external magnet [13] [12]. | Synthesis of core magnetic nanoparticles. |
| Sodium Tripolyphosphate (TPP) | Cross-linking agent; enhances mechanical stability in acidic media [68] [13]. | Preparation of TPP-CMN nano-sorbents [13]. |
| Ethylenediaminetetraacetic Acid (EDTA) | Chelating agent; functionalizes composite to enhance metal uptake capacity [69]. | Synthesis of EDTA-M-Cs composite [69]. |
| Lignin | Biopolymer co-adsorbent; introduces additional functional groups to create a heterogeneous surface [29]. | Creation of chitosan-lignin biocomposite [29]. |
| Vanillin | Cross-linking and modifying agent; can enhance adsorption properties [13]. | Synthesis of V-CMN nano-sorbents [13]. |
| Potassium Dichromate (K₂Cr₂O₇) | Source of toxic Cr(VI) ions in adsorption experiments [29] [12]. | Testing adsorbent performance for chromium removal. |
| 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide (EDC) | Cross-linker for covalent bonding between molecules. | (Commonly used, implied in functionalization). |
| N-Hydroxysuccinimide (NHS) | Often used with EDC to improve coupling efficiency. | (Commonly used, implied in functionalization). |
| Acetic Acid | Solvent for dissolving chitosan [68] [29]. | Preparation of chitosan solutions for composite synthesis. |
| Sodium Hydroxide (NaOH) | pH adjustment; precipitation agent [29] [13]. | Used in synthesis and to maintain optimal pH for adsorption. |
Optimizing the critical operational parameters—pH, dosage, contact time, and temperature—is essential for harnessing the full potential of nanomagnetic chitosan composites in heavy metal remediation. The comparative data presented reveals that while universal optimums are elusive, strong trends exist. pH is a primary governor of adsorption mechanisms, often with low pH favoring cationic and high pH favoring anionic metal removal. Dosage must be optimized to balance efficiency with cost, while equilibrium time can be remarkably fast (15-30 minutes) for some advanced composites. The effect of temperature can be either endothermic or exothermic, depending on the specific composite-pollutant pair. Employing systematic experimental protocols like batch studies and RSM is crucial for efficient and reliable optimization, paving the way for the successful application of these promising materials in addressing global water pollution challenges.
Nanomagnetic chitosan composites represent a significant advancement in materials science, combining the excellent adsorption properties of the biopolymer chitosan with the magnetic separation capabilities of iron oxide nanoparticles. These hybrid materials have shown tremendous promise in environmental remediation, particularly for the removal of heavy metals from wastewater [70]. However, their practical application faces several material limitations, including mechanical instability under flow conditions, susceptibility to acidic degradation, and nanoparticle aggregation, which can reduce active surface area and overall efficiency [71]. This guide objectively compares the performance of various nanomagnetic chitosan composites, focusing on strategies to overcome these limitations while providing supporting experimental data and methodologies to aid researchers in selecting and developing optimal materials for heavy metal removal applications.
Comparative Performance of Nanomagnetic Chitosan Composites
The development of nanomagnetic chitosan composites has progressed significantly, with various synthesis methods and modifications yielding materials with distinct structural characteristics and performance capabilities. The choice of synthesis strategy directly impacts the resulting composite's properties, including its mechanical integrity, stability across different pH conditions, and tendency toward particle aggregation.
Table 1: Comparison of Synthesis Methods for Nanomagnetic Chitosan Composites
| Synthesis Method | Key Features | Particle Size Range | Mechanical Stability | pH Stability | Aggregation Tendency |
|---|---|---|---|---|---|
| Two-Step Coprecipitation | Sequential synthesis: magnetic NPs first, then chitosan coating | 10-100 nm [72] | Moderate | Broad [72] | Moderate to High |
| One-Step Coprecipitation | Simultaneous formation of magnetic NPs in chitosan solution [73] | 4.5-50 nm [73] | Good | Limited in strong acids [71] | Low |
| Microemulsion Template | Reverse micelles control nanoparticle size and distribution [73] | 4.5-10 nm [73] | Excellent | Good | Very Low |
| Cross-Linked Composites | Chemical cross-linkers (e.g., glutaraldehyde) enhance structural integrity [72] | 20-80 nm [72] | Excellent | Enhanced acidic stability [71] | Low |
The synthesis methodology significantly influences the final composite properties. The microemulsion template approach produces exceptionally uniform nanoparticles with minimal aggregation due to the confined reaction environment within reverse micelles [73]. Meanwhile, cross-linking strategies substantially improve mechanical strength and pH stability by creating covalent bonds between polymer chains, preventing dissolution in acidic environments [72] [71].
Heavy Metal Removal Efficiency: Comparative Experimental Data
The efficacy of various nanomagnetic chitosan composites in removing heavy metals from aqueous solutions has been extensively studied. Performance varies significantly based on composite composition, surface functionalization, and experimental conditions.
Table 2: Heavy Metal Removal Efficiency of Different Chitosan Composites
| Composite Type | Target Heavy Metal | Experimental Conditions | Removal Efficiency/ Capacity | Key Advantages |
|---|---|---|---|---|
| Chitosan-coated γ-Fe₂O₃ | Pb²⁺ [73] | 10 min treatment, aqueous solution | Complete removal in 10 min [73] | Rapid kinetics, high productivity |
| Chitosan-Zeolite Composite | Cu(II), Cd(II), Pb(II) [74] | Not specified | High efficiency [74] | Enhanced surface area, multiple binding sites |
| Magnetic Chitosan Nanoparticles | Various heavy metals [34] | Not specified | >400 mg g⁻¹ capacity [34] | High adsorption capacity, superparamagnetism |
| Aminated Chitosan Composite | Cr(VI) [74] | Not specified | Effective removal [74] | Specific functionalization for targeted metals |
| Chitosan-EDTA Composite | Various heavy metals [70] | Not specified | Enhanced capacity [70] | Improved chelation properties |
The data reveal that composite adsorbents consistently outperform pure chitosan materials, with specialized functionalization further enhancing removal capabilities for specific metal ions. The abundance of amino and hydroxyl groups in chitosan provides numerous binding sites for metal ions through mechanisms including chelation, ion exchange, and electrostatic attraction [74]. Engineered nanocomposites leverage these inherent properties while incorporating additional functional groups and structural enhancements to achieve superior performance.
Experimental Protocols for Composite Synthesis and Evaluation
One-Step Coprecipitation Synthesis
This efficient method enables simultaneous formation of magnetic nanoparticles and chitosan coating in a single reaction vessel [73]:
- Step 1: Prepare a 0.25 M aqueous solution containing FeCl₃·6H₂O and FeCl₂·4H₂O in a 2:1 molar ratio.
- Step 2: Dissolve medium molecular weight chitosan (degree of deacetylation 75-85%) in dilute acetic acid (1-2% v/v) to create a 0.1-1% (w/v) polymer solution.
- Step 3: Combine the iron solutions with chitosan solution under vigorous stirring at 80°C.
- Step 4: Adjust pH to 9-11 using ammonium hydroxide or sodium hydroxide solution to precipitate the composite nanoparticles.
- Step 5: Maintain reaction at 80°C for 2 hours with continuous stirring under nitrogen atmosphere.
- Step 6: Separate products magnetically, wash repeatedly with distilled water and ethanol, and dry at 50°C overnight.
Composite Cross-Linking Protocol
Chemical cross-linking significantly enhances mechanical and pH stability [72]:
- Step 1: Suspend pre-formed chitosan-magnetic nanoparticles in aqueous solution.
- Step 2: Add cross-linking agent (e.g., 0.5-2.5% glutaraldehyde or pentaethylenehexamine) dropwise with constant stirring.
- Step 3: Adjust pH to alkaline conditions (pH 9-10) using dilute NaOH.
- Step 4: React for 2-4 hours at ambient temperature or elevated temperatures (50-60°C).
- Step 5: Recover cross-linked nanoparticles magnetically and wash thoroughly to remove unreacted cross-linker.
- Step 6: Dry under vacuum or freeze-dry for storage.
Adsorption Capacity Evaluation
Standard methodology for quantifying heavy metal removal efficiency [70]:
- Step 1: Prepare stock solutions of target heavy metals (e.g., Pb²⁺, Cu²⁺, Cd²⁺) at 1000 mg/L in deionized water.
- Step 2: Add known quantities of nanocomposite (10-50 mg) to metal solutions of varying concentrations (10-500 mg/L).
- Step 3: Agitate mixtures at constant temperature (e.g., 25°C) for predetermined time intervals (5 min to 24 h).
- Step 4: Separate adsorbent magnetically and analyze supernatant for residual metal concentration using AAS or ICP-MS.
- Step 5: Calculate adsorption capacity using the formula: ( qe = \frac{(C0 - Ce) \times V}{m} ) where ( qe ) = adsorption capacity (mg/g), ( C0 ) and ( Ce ) = initial and equilibrium concentrations (mg/L), V = solution volume (L), and m = adsorbent mass (g).
Structural and Mechanistic Insights
The superior performance of engineered chitosan composites stems from their optimized nanostructure, which provides increased specific surface area, enhanced porosity, and a dense array of reactive functional groups [34]. These structural characteristics facilitate multiple interaction mechanisms with heavy metal ions while simultaneously improving mass transfer kinetics.
The diagram illustrates the multiple mechanisms through which chitosan composites interact with heavy metal ions. The amino and hydroxyl groups present in chitosan serve as primary binding sites, participating in various interactions including chelation, ion exchange, and electrostatic attraction [74]. Composites enhance these inherent properties through increased surface area and additional functionalization, enabling more efficient metal ion capture.
The Scientist's Toolkit: Essential Research Reagents and Materials
Successful development and evaluation of nanomagnetic chitosan composites requires specific reagents and characterization tools. The following table details essential materials and their functions in composite synthesis and testing.
Table 3: Essential Research Reagents and Materials for Composite Development
| Material/Reagent | Specifications | Function in Research | Example Applications |
|---|---|---|---|
| Chitosan | Medium molecular weight, degree of deacetylation 75-85% [72] | Primary biopolymer matrix providing functional groups | Base material for all composite variants [72] |
| Iron Precursors | FeCl₃·6H₂O and FeCl₂·4H₂O (2:1 molar ratio) [73] | Source of iron for magnetic nanoparticle formation | Coprecipitation synthesis [73] |
| Cross-Linking Agents | Glutaraldehyde, pentaethylenehexamine (PEHA) [72] | Enhance mechanical strength and pH stability | Stabilizing composites for acidic applications [72] |
| Characterization Tools | XRD, FT-IR, VSM, SEM/TEM [33] [43] | Structural, magnetic, and morphological analysis | Confirming composite formation and properties [33] |
| Heavy Metal Salts | Pb(NO₃)₂, CuSO₄, CdCl₂, K₂Cr₂O₇ | Preparation of standard solutions for adsorption tests | Evaluating removal capacity and efficiency [70] |
The selection of appropriate chitosan specifications is particularly critical, as the degree of deacetylation directly influences the availability of amino groups for metal binding and cross-linking reactions [74]. Similarly, the purity and ratio of iron precursors significantly impact the crystalline structure and magnetic properties of the resulting nanoparticles [73].
The strategic development of nanomagnetic chitosan composites has successfully addressed key material limitations while enhancing heavy metal removal capabilities. Comparative analysis demonstrates that cross-linked composites provide superior mechanical strength and pH stability, while microemulsion-synthesized materials exhibit minimal aggregation tendencies. The selection of appropriate synthesis methods and functionalization strategies enables researchers to tailor composite properties for specific application requirements. As research advances, focus remains on optimizing the balance between adsorption capacity, structural stability, and practical implementation factors to enable scalable wastewater treatment solutions. The continued refinement of these composite materials holds significant promise for addressing global challenges in water purification and environmental remediation.
The treatment of industrial wastewater containing multiple heavy metal ions presents a significant scientific and engineering challenge. Unlike idealized single-metal systems, multi-metal matrices involve complex interactions where different ions compete for adsorption sites, a phenomenon known as competitive adsorption [41]. This comparative analysis examines the efficacy of various nanomagnetic chitosan composites in managing these complex scenarios, focusing on their removal efficiency, selectivity, and binding capacity for heavy metal ions in multi-component systems. The integration of magnetic properties with the versatile adsorption capabilities of chitosan creates a promising class of versatile adsorbents that can be efficiently separated and reused [47]. Understanding the interplay between different metal ions and their collective impact on adsorption performance is crucial for developing effective wastewater treatment strategies for real-world industrial applications where multiple contaminants coexist.
Performance Comparison of Nanomagnetic Chitosan Composites
The efficacy of magnetic chitosan composites varies significantly depending on their composition, synthesis method, and target heavy metals. The table below provides a comparative overview of different composites and their documented performance in heavy metal removal.
Table 1: Performance comparison of magnetic chitosan composites for heavy metal removal
| Composite Type | Target Heavy Metals | Maximum Adsorption Capacity (mg/g) | Equilibrium Time (min) | Key Advantages | Reference |
|---|---|---|---|---|---|
| Magnetic Chitosan Composite (MCC) | Pb(II) | 220.9 | 120 | High adsorption capacity for lead, easy magnetic separation | [5] [6] |
| Magnetic Chitosan Composite (MCC) | Cu(II) | 216.8 | 120 | Excellent copper uptake, versatile for wastewater treatments | [5] [6] |
| Magnetic Chitosan Composite (MCC) | Ni(II) | 108.9 | 120 | Good nickel removal, magnetically recoverable | [5] [6] |
| Chitosan-Clay-Magnetite | Cu(II), As(V) | Not specified | Not specified | Effective for concurrent copper and arsenic removal | [5] |
| Magnetic Chitosan-Tripolyphosphate with Silica Coating | Cu(II) | Not specified | Not specified | Enhanced stability and adsorption properties | [5] |
The data reveals that magnetic chitosan composites exhibit particularly strong affinity for lead and copper ions, with capacities exceeding 200 mg/g for these metals [5] [6]. The competitive adsorption dynamics in multi-metal systems show that selectivity often follows the order Pb(II) > Cu(II) > Ni(II), reflecting the varying binding strengths of different metals to the chitosan functional groups [5]. This hierarchy is influenced by factors such as ionic radius, electronegativity, and coordination chemistry with the amino and hydroxyl groups present in chitosan [47].
Experimental Protocols for Evaluating Competitive Adsorption
Composite Synthesis Methods
The coprecipitation method represents one of the most common approaches for synthesizing magnetic chitosan composites. In this protocol, Fe²⁺ and Fe³⁺ ions are typically co-precipitated in an alkaline medium in the presence of chitosan to form magnetite (Fe₃O₄) nanoparticles embedded within the chitosan matrix [47]. The two-step method involves first synthesizing Fe₃O₄ magnetic nanoparticles separately, then encapsulating them with chitosan [47]. Alternative synthesis techniques include crosslinking methods using agents such as glutaraldehyde or epichlorohydrin to enhance mechanical stability, spray drying for producing uniform composite microspheres, and electrostatic drop method for generating well-defined beads [47].
Adsorption Experiments in Multi-Metal Systems
Standard experimental protocols for evaluating competitive adsorption involve preparing multi-metal solutions with varying initial concentrations of target contaminants such as Pb(II), Cu(II), and Ni(II) [5]. The pH is carefully adjusted using dilute NaOH or HNO₃ solutions, as pH significantly influences metal speciation and chitosan's surface charge [47]. Batch adsorption experiments are conducted by adding a fixed dose of the magnetic chitosan composite to the multi-metal solution under constant agitation. Samples are extracted at predetermined time intervals, separated using an external magnet, and the supernatant is analyzed via inductively coupled plasma optical emission spectrometry (ICP-OES) or atomic absorption spectroscopy (AAS) to determine residual metal concentrations [75].
Table 2: Key parameters in competitive adsorption experiments
| Parameter | Typical Range | Impact on Adsorption |
|---|---|---|
| pH | 3.0-6.0 | Critical for metal speciation and chitosan protonation |
| Temperature | 25-45°C | Affects kinetics and thermodynamic parameters |
| Initial Concentration | 50-500 mg/L | Influences adsorption capacity and competition dynamics |
| Adsorbent Dose | 0.5-2.0 g/L | Impacts removal efficiency and available sites |
| Contact Time | 10-180 min | Determines equilibrium attainment and kinetics |
The adsorption capacity for each metal in mixed systems is calculated using the formula:
[qe = \frac{(C0 - C_e) \times V}{m}]
where (qe) is the adsorption capacity (mg/g), (C0) and (Ce) are initial and equilibrium concentrations (mg/L), respectively, (V) is the solution volume (L), and (m) is the adsorbent mass (g) [75]. In competitive systems, the selectivity coefficient for metal A over metal B can be calculated as ((q{e,A}/C{e,A})/(q{e,B}/C{e,B})), where (q{e,A}) and (q{e,B}) are equilibrium adsorption capacities, and (C{e,A}) and (C_{e,B}) are equilibrium concentrations for metals A and B, respectively [41].
Mechanisms of Competitive Adsorption and Ion Interference
The adsorption of heavy metals to magnetic chitosan composites primarily occurs through coordination with amino (–NH₂) and hydroxyl (–OH) groups, ion exchange, and electrostatic interactions [47]. In multi-metal systems, competition arises because these functional groups have varying affinities for different metal ions. The interference patterns observed in competitive adsorption are influenced by several factors:
Ionic Characteristics: Metals with higher electronegativity, smaller hydrated ionic radius, and higher charge density typically exhibit stronger binding to chitosan functional groups [41]. This explains the commonly observed selectivity sequence where Pb(II) often outcompetes Cu(II), which in turn shows higher affinity than Ni(II) [5].
Solution Chemistry: pH significantly affects both the speciation of metal ions and the surface charge of the adsorbent. The point of zero charge (pHPZC) of magnetic chitosan composites typically ranges between 8.0-8.5, meaning the surface is positively charged at lower pH values and negatively charged at higher pH values [5]. This influences electrostatic interactions with metal cations.
Steric Factors: The spatial arrangement of functional groups and the accessibility of binding sites create steric preferences for certain metal ions over others, contributing to selectivity in multi-metal systems [47].
The diagram below illustrates the competitive adsorption mechanism in multi-metal systems:
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 3: Essential research reagents for magnetic chitosan adsorption studies
| Reagent/Material | Function/Application | Typical Specifications |
|---|---|---|
| Chitosan | Primary adsorbent matrix providing functional groups | Degree of deacetylation >75%, medium molecular weight |
| FeCl₂·4H₂O | Iron source for magnetic nanoparticle synthesis | Analytical grade, ≥99% purity |
| FeCl₃·6H₂O | Iron source for magnetic nanoparticle synthesis | Analytical grade, ≥98% purity |
| NH₄OH | Precipitation agent for magnetite formation | 25-28% aqueous solution |
| Glutaraldehyde | Crosslinking agent for enhanced stability | 25% aqueous solution, electron microscopy grade |
| Pb(NO₃)₂ | Lead ion source for adsorption studies | Analytical grade, ≥99% purity |
| CuSO₄·5H₂O | Copper ion source for adsorption studies | Analytical grade, ≥99% purity |
| NiCl₂·6H₂O | Nickel ion source for adsorption studies | Analytical grade, ≥98% purity |
| NaOH/HNO₃ | pH adjustment solutions | 0.1M solutions in deionized water |
The experimental workflow for preparing and evaluating magnetic chitosan composites involves multiple stages, as illustrated below:
The comparative analysis of nanomagnetic chitosan composites reveals their significant potential for managing complex multi-metal matrices in wastewater treatment. While these composites demonstrate versatile adsorption capabilities across various heavy metals, their performance in competitive systems shows distinct selectivity patterns favoring Pb(II) and Cu(II) over Ni(II) [5] [6]. The competitive adsorption dynamics are influenced by multiple factors including ionic characteristics, solution chemistry, and composite structure [41]. The integration of magnetic properties enables efficient separation and potential reusability, addressing practical implementation challenges [47]. Future research directions should focus on enhancing selectivity for specific metal ions through targeted functionalization, improving structural stability for long-term application, and validating performance in real industrial wastewater scenarios with complex matrices. The development of predictive models for competitive adsorption behavior will be crucial for optimizing system design and operation parameters for specific multi-metal contamination scenarios.
The removal of heavy metals from wastewater using nanomagnetic chitosan composites represents a significant advancement in adsorption technology. However, the practical implementation of these materials depends critically on their regeneration potential and reusability performance over multiple operational cycles. While adsorption simply transfers contaminants from wastewater to the solid adsorbent surface, regeneration completes the treatment cycle by recovering both the adsorbed species and the adsorption capacity of the material, thereby addressing the secondary pollution concern and improving economic viability [76]. This comparative guide examines the desorption strategies and long-term performance of various nanomagnetic chitosan composites, providing researchers with critical insights for selecting and optimizing these materials for sustainable wastewater treatment applications.
Performance Comparison of Nanomagnetic Chitosan Composites
The regeneration efficiency and reusability of nanomagnetic chitosan composites vary significantly based on their structural composition, functionalization approaches, and desorption protocols. The table below summarizes the comparative performance of different composite types based on current research findings.
Table 1: Comparative Performance of Nanomagnetic Chitosan Composites for Heavy Metal Removal
| Composite Type | Functionalization | Maximum Adsorption Capacity | Optimal Desorption Agent | Regeneration Efficiency | Reusability Cycles |
|---|---|---|---|---|---|
| Chitosan-Lignin Biocomposite | Non-covalent blending | 52.06-72.61 mg/g for Cr(VI) | Acidic solutions (pH~2) | High (exothermic physisorption) | Data not specified [29] |
| Magnetic Chitosan/CNT | Thiol-functionalized | Not specified | Not specified | Moderate | 4 cycles [77] |
| Magnetic Chitosan/CNT | Non-functionalized | Not specified | Not specified | High | 27 cycles [77] |
| Nano-chitosan based composites | Various modifications | >400 mg/g for various metals | Acidic/chelating agents | High | Varies by modification [34] |
Experimental Protocols for Regeneration Studies
Adsorption-Desorption Cycle Methodology
Standardized experimental protocols are essential for obtaining comparable data on regeneration efficiency and reusability. The following methodology represents common practices in the field:
Saturation Phase: Adsorbents are saturated with target heavy metal ions (e.g., Cr(VI)) using optimized parameters from adsorption isotherms (pH, contact time, temperature) [29].
Separation Phase: Magnetic composites are separated using an external magnetic field, eliminating the need for filtration or centrifugation [77].
Desorption Phase: Various desorbing agents are tested, including:
Regeneration Phase: The desorbed adsorbent is washed with distilled water and reactivated using mild drying (60-100°C for 2-3 hours) before subsequent cycles [29].
Analysis Phase: The desorption efficiency is calculated using the formula:
Desorption Efficiency (%) = (Amount of Metal Desorbed / Amount of Metal Adsorbed) × 100
Adsorption capacity retention is monitored across multiple cycles to determine reusability potential [78].
Composite-Specific Testing Protocols
For chitosan-lignin biocomposites, experiments are typically conducted at acidic pH (approximately 2.0 for Cr(VI)) with continuous stirring at 240 rpm, using 0.1 g adsorbent in 50 mL metal solution [29]. For magnetic chitosan/carbon nanotube composites, the focus shifts to structural stability under different pH conditions (optimal pH 3.5 for immobilized laccase systems) and temperature ranges (optimal 45°C) [77].
Regeneration Mechanisms and Strategic Approaches
The regeneration of spent adsorbents operates through multiple mechanistic pathways, each requiring specific desorption strategies:
Ion Exchange Mechanism
In chitosan-based composites where heavy metal removal occurs primarily through ion exchange with protonated amino groups, acid treatment effectively regenerates the material by reversing this exchange process. Dilute acidic solutions (0.1-0.5 M) protonate the adsorption sites, displacing the bound metal cations back into solution [76] [78].
pH-Dependent Desorption
For heavy metals like chromium that exhibit pH-dependent speciation and adsorption affinity, simple pH adjustment serves as an effective regeneration method. The chitosan-lignin biocomposite demonstrates optimal Cr(VI) adsorption at acidic pH (~2), while desorption occurs effectively at alkaline conditions through hydroxide competition [29].
Chemical Elution
Chelating agents such as EDTA form stable complexes with metal ions, effectively stripping them from adsorption sites through competitive coordination. This approach is particularly effective for composites where chemisorption contributes significantly to metal uptake [76].
Magnetic Recovery Advantage
Nanomagnetic composites incorporating Fe₃O₄ nanoparticles enable efficient solid-liquid separation after each adsorption-desorption cycle using external magnetic fields. This preserves structural integrity compared to conventional filtration, significantly enhancing operational lifespan [77].
Visualization of the cyclic regeneration workflow for nanomagnetic chitosan composites, highlighting the key stages from spent adsorbent to performance assessment for subsequent reuse.
Factors Influencing Regeneration Efficiency and Long-Term Stability
Material Composition and Structural Integrity
The fundamental composition of chitosan composites directly determines their regeneration potential. Non-functionalized magnetic chitosan/CNT composites demonstrate exceptional reusability (27 cycles) compared to their thiol-functionalized counterparts (4 cycles), attributed to the breakdown of unstable disulfide bonds in functionalized materials [77]. Chitosan-lignin biocomposites maintain performance through non-covalent interactions that preserve functional group accessibility across cycles [29].
Desorption Agent Selection
The choice of desorption agent significantly impacts regeneration efficiency:
- Acidic eluents (HCl, HNO₃) effectively protonate adsorption sites, reversing ion exchange processes.
- Alkaline solutions (NaOH) compete with anionic metal species for adsorption sites.
- Chelating agents (EDTA) form stable complexes, physically stripping metals from surfaces.
- Salt solutions (NaCl, NaNO₃) utilize high ionic strength to displace adsorbed metals [76] [78].
Operational Parameters
Critical operational factors include:
- Contact time during desorption must be sufficient to reach equilibrium.
- Temperature influences desorption kinetics and efficiency, with chitosan-lignin composites exhibiting exothermic adsorption (more easily desorbed at higher temperatures) [29].
- Eluent concentration must balance effectiveness with material preservation.
- Solid-liquid ratio during desorption affects concentration gradients and efficiency.
The Researcher's Toolkit: Essential Materials and Reagents
Table 2: Essential Research Reagents for Regeneration Studies
| Reagent/Category | Specific Examples | Primary Function in Regeneration |
|---|---|---|
| Acidic Eluents | HCl, HNO₃, CH₃COOH (0.1-0.5 M) | Protonate adsorption sites, reverse ion exchange |
| Basic Eluents | NaOH, NH₄OH (0.1-0.5 M) | Compete with anionic metal species |
| Salt Solutions | NaCl, NaNO₃ | Displace metals through ionic strength |
| Chelating Agents | EDTA, citric acid | Form stable complexes with metal ions |
| Organic Solvents | Ethanol, methanol | Remove organic contaminants, regenerate pores |
| Magnetic Components | Fe₃O₄ nanoparticles | Enable efficient solid-liquid separation |
| Composite Matrix | Chitosan polymer base | Provide primary adsorption sites |
| Crosslinkers | Glutaraldehyde | Enhance structural stability across cycles |
| Functionalization Agents | Cysteamine (thiolation) | Introduce specific metal-binding groups |
Comparative Analysis and Research Implications
The regeneration potential and reusability of nanomagnetic chitosan composites present a complex landscape with significant implications for research and application:
Performance Trade-offs
A clear trade-off emerges between adsorption capacity and regeneration potential. While functionalized composites often demonstrate enhanced initial adsorption, their regeneration potential may be compromised by the instability of functional groups under repeated cycles. Non-functionalized magnetic chitosan/CNT composites exhibit remarkable reusability (27 cycles) despite potentially lower initial capacity [77].
Structural Stability Considerations
Composites maintaining performance across multiple cycles share common characteristics: robust mechanical stability, preserved magnetic responsiveness, and maintained structural integrity after chemical treatment. Chitosan-lignin biocomposites demonstrate this through stable performance attributed to their heterogeneous monolayer structure with two distinct functional groups [29].
Economic and Environmental Implications
From a practical implementation perspective, regeneration efficiency directly influences treatment cost and environmental impact. Composites capable of multiple regeneration cycles with minimal capacity loss (≤20% after 5 cycles) offer significantly better life-cycle economics and reduced waste generation compared to single-use adsorbents [76] [78].
The regeneration and reusability of nanomagnetic chitosan composites for heavy metal removal depend critically on the interplay between material design, desorption strategy selection, and operational parameters. Current research indicates that while functionalization can enhance initial adsorption capacity, it may compromise long-term reusability due to structural instability. Non-functionalized composites often demonstrate superior performance retention across multiple cycles, highlighting the importance of balancing capacity with durability. Future research directions should focus on developing standardized testing protocols, exploring novel regeneration approaches, and conducting comprehensive life-cycle assessments to advance these promising materials toward practical implementation in wastewater treatment applications.
The contamination of aquatic environments by heavy metals and dyes poses a critical threat to ecosystems and human health, necessitating the development of effective and sustainable remediation strategies [34]. Among various treatment technologies, adsorption is recognized as a highly promising method due to its simple operation, low cost, high removal efficiency, and environmental friendliness [47]. In recent decades, nano-chitosan composites have emerged as frontier adsorbents that combine the exceptional properties of chitosan biopolymers with the functionality of nanoscale materials and magnetic components [34] [47].
Chitosan, derived from the deacetylation of chitin found in crustacean shells, possesses unique advantages for water treatment applications, including abundant functional groups (amino and hydroxyl), biodegradability, biocompatibility, and environmental safety [79] [47]. However, native chitosan has limitations such as pH sensitivity, weak mechanical properties, and difficulty in separation from treated water [47]. These challenges have driven the development of modified chitosan materials, particularly magnetic chitosan composites that incorporate magnetic nanoparticles (especially Fe₃O₄), enabling efficient separation using external magnetic fields while enhancing adsorption capacity and mechanical strength [47].
This review provides a comprehensive comparison of different nanomagnetic chitosan composites for heavy metal removal, examining their synthesis methods, adsorption performance, operational mechanisms, and cost-benefit considerations within the framework of eco-design principles that balance high efficiency with economic and environmental sustainability.
Experimental Protocols for Composite Synthesis and Evaluation
Synthesis Methods for Magnetic Chitosan Composites
The preparation of magnetic chitosan composites typically follows two main approaches: the coprecipitation method and the crosslinking method [47]. The coprecipitation method can be further divided into in situ and two-step processes, each with distinct advantages for creating composites with specific characteristics.
The in situ coprecipitation method involves forming magnetic nanoparticles within a chitosan matrix. A typical protocol involves first dissolving chitosan in dilute acetic acid to create a homogeneous solution. Ferric (Fe³⁺) and ferrous (Fe²⁺) ions in a 2:1 molar ratio are then added to the chitosan solution with continuous stirring. The mixture is then alkalized using sodium hydroxide or ammonia to precipitate Fe₃O₄ nanoparticles within the polymer matrix [47]. This one-pot synthesis approach ensures uniform distribution of magnetic particles throughout the composite structure. Jumadi et al. enhanced this method by incorporating sodium dodecyl sulfate to partially reduce Fe³⁺ to Fe²⁺, facilitating nanoparticle formation [47].
The two-step coprecipitation method involves pre-forming magnetic nanoparticles before incorporating them into the chitosan matrix. In this approach, Fe₃O₄ nanoparticles are first synthesized separately via chemical coprecipitation, hydrothermal methods, or other techniques. These pre-formed nanoparticles are then mixed with chitosan solution, often using crosslinking agents such as glutaraldehyde or epichlorohydrin to stabilize the composite structure [47]. This method provides better control over magnetic nanoparticle size and crystallinity but requires additional processing steps.
For creating specialized composite structures, researchers have employed additional techniques such as the encapsulation method described by Yuan et al., where chitosan solution mixed with magnetic nanoparticles is sprayed into a crosslinking solution (e.g., 5% NaOH) through a nozzle to form uniform beads [80]. The resulting beads can be further modified with polyethylenimine (PEI) and glutaraldehyde to enhance their functionality for specific applications such as enzyme immobilization or heavy metal adsorption [80].
Adsorption Performance Evaluation Protocols
Standardized experimental protocols are essential for comparing the performance of different magnetic chitosan composites. A typical adsorption experiment involves preparing stock solutions of target contaminants (heavy metals or dyes) at known concentrations, then diluting to desired concentrations for testing [29].
In a representative study examining chitosan-lignin biocomposite for removal of Reactive Orange 16 (RO16) dye and Cr(VI), researchers used the following protocol: 0.1 g of adsorbent was added to 50 mL of contaminant solution at varying concentrations (10-100 mg/L). The mixture was stirred continuously at 240 rpm at controlled temperatures (25, 35, and 45°C) [29]. For Cr(VI) adsorption, pH was adjusted to 2 using HCl or NaOH, as chitosan-based composites typically achieve maximum Cr(VI) adsorption under acidic conditions [29]. Samples were collected at regular intervals, centrifuged at 3400 rpm, and analyzed using UV-Vis spectrophotometry (580 nm for RO16, 545 nm for Cr(VI) complexed with 1,5-diphenylcarbazide) [29].
Adsorption capacity calculations typically follow the equation: [ qe = \frac{(C0 - Ce) \times V}{m} ] where ( qe ) is the adsorption capacity (mg/g), ( C0 ) and ( Ce ) are initial and equilibrium contaminant concentrations (mg/L), V is solution volume (L), and m is adsorbent mass (g) [29].
The experimental workflow for developing and evaluating magnetic chitosan composites follows a systematic process from material synthesis to performance assessment, as illustrated below:
Experimental Workflow for Composite Development
Comparative Performance of Nanomagnetic Chitosan Composites
Adsorption Capacity Comparison
The adsorption performance of magnetic chitosan composites varies significantly based on their specific composition, modification methods, and target contaminants. The table below provides a comparative analysis of different composite types and their effectiveness for heavy metal and dye removal:
Table 1: Comparison of Adsorption Performance for Different Magnetic Chitosan Composites
| Composite Type | Target Contaminant | Maximum Adsorption Capacity (mg/g) | Optimal pH | Reference |
|---|---|---|---|---|
| Chitosan + 50% Lignin Biocomposite | RO16 Dye | 79.76 mg/g | 6 | [29] |
| Chitosan + 50% Lignin Biocomposite | Cr(VI) | 72.61 mg/g | 2 | [29] |
| Chitosan-Magnetic (Fe₃O₄) | Cu(II) | Varies by modification: 27.5-188.7 mg/g | ~5-6 | [47] |
| Chitosan-Magnetic (Fe₃O₄) | Pb(II) | Varies by modification: 32.1-263.2 mg/g | ~5-6 | [47] |
| Chitosan-Magnetic (Fe₃O₄) | Cr(VI) | Varies by modification: 35.8-245.3 mg/g | ~2 | [47] |
| Chitosan-Magnetic Nanocomposite | Various Dyes | Up to 400 mg/g | Varies by dye type | [34] |
The exceptional performance of these composites stems from their engineered nanostructure, which increases specific surface area, enhances porosity, and provides a dense array of reactive functional groups [34]. This structural optimization facilitates electrostatic interactions, chemical bonding, and improved mass transfer, leading to faster adsorption kinetics, higher equilibrium uptake, and robust recyclability [34].
The chitosan-lignin biocomposite demonstrates particularly interesting properties, with statistical physics modeling revealing that RO16 and Cr(VI) interact with two distinct functional groups on the chitosan + 50% lignin surface [29]. The adsorption energies ranged from 4.88 to 16.97 kJ/mol, consistent with physisorption mechanisms dominated by hydrogen bonding and electrostatic interactions [29].
Economic and Environmental Sustainability Assessment
The eco-design of magnetic chitosan composites must balance adsorption performance with economic viability and environmental sustainability. The table below compares key sustainability metrics across different composite types:
Table 2: Economic and Environmental Sustainability Indicators for Chitosan Composites
| Parameter | Basic Chitosan | Magnetic Chitosan | Chitosan-Lignin Biocomposite | Commercial Activated Carbon |
|---|---|---|---|---|
| Raw Material Cost | Low (from seafood waste) | Low to Moderate | Very Low (lignin from agricultural waste) | High |
| Separation Cost | High (filtration/centrifugation) | Very Low (magnetic separation) | Low to Moderate | Moderate |
| Regeneration Potential | Good (5-10 cycles) | Excellent (10+ cycles) | Good (5-8 cycles) | Limited (3-5 cycles) |
| Environmental Impact | Low (biodegradable) | Low to Moderate | Very Low (fully bio-based) | Moderate (energy-intensive production) |
| Adsorption Capacity | Moderate | High | High to Very High | High |
| Implementation Scale | Lab to Pilot Scale | Lab to Pilot Scale | Lab Scale | Full Commercial Scale |
The sustainability advantages of chitosan-based composites are multifaceted. As noted in Rhoades Hall case study on sustainable design, renovations and material choices that prioritize lifecycle thinking deliver tremendous return on investment through reduced operational costs and environmental impact [81]. Similarly, magnetic chitosan composites exemplify the circular economy principles by valorizing waste materials (chitosan from crustacean shells, lignin from agricultural residues) into high-value water treatment applications [29] [14].
The magnetic separation capability of these composites provides significant economic advantages by reducing operational costs associated with solid-liquid separation. As highlighted by Shaumbwa et al., magnetic separation techniques enable rapid, efficient recovery of adsorbents without requiring additional filtration or centrifugation steps [47]. This translates to reduced energy consumption and lower operational expenses in water treatment processes.
Adsorption Mechanisms and Functional Performance
Molecular-Level Interactions
The superior adsorption performance of nanomagnetic chitosan composites originates from their multifunctional active sites and engineered nanostructure. The primary mechanisms include:
Electrostatic Interactions: Protonated amino groups (-NH₃⁺) on chitosan backbone attract anionic pollutants such as Cr(VI) oxyanions (HCrO₄⁻, CrO₄²⁻) and anionic dyes [29] [47]. This mechanism is highly pH-dependent, with optimal Cr(VI) removal occurring at pH 2 where amine protonation is maximized [29].
Surface Complexation: Heavy metal cations (Cu²⁺, Pb²⁺, Cd²⁺) form coordination complexes with amino and hydroxyl groups on chitosan, facilitated by lone pair electron donation [47]. Modified composites with additional functional groups (carboxyl, sulfonate, etc.) enhance this mechanism through chelation.
Hydrogen Bonding: Hydroxyl and amino groups form hydrogen bonds with various pollutant molecules, particularly dyes containing hydroxyl, amino, or carbonyl functional groups [29]. In chitosan-lignin composites, the abundant phenolic hydroxyl groups on lignin significantly contribute to this mechanism.
Physical Adsorption: The high surface area and nanoporous structure of composites provide extensive surfaces for physisorption of contaminants through van der Waals forces and pore filling [34] [47].
The following diagram illustrates the primary adsorption mechanisms at the molecular level:
Adsorption Mechanisms at Molecular Level
The Scientist's Toolkit: Essential Research Reagents and Materials
The development and evaluation of nanomagnetic chitosan composites requires specific reagents and characterization tools. The following table details essential materials and their functions in composite synthesis and testing:
Table 3: Essential Research Reagents and Materials for Composite Development
| Reagent/Material | Specification | Primary Function | Application Notes |
|---|---|---|---|
| Chitosan | Medium molecular weight (50-90 kDa), Deacetylation degree ≥80% | Primary adsorbent matrix providing functional groups | Soluble in dilute acetic acid; degree of deacetylation affects functionality |
| Iron Precursors | FeCl₃·6H₂O (Ferric), FeCl₂·4H₂O (Ferrous) | Magnetic nanoparticle (Fe₃O₄) synthesis | Maintain 2:1 Fe³⁺:Fe²⁺ ratio for optimal magnetite formation |
| Crosslinking Agents | Glutaraldehyde (25-50%), Epichlorohydrin | Stabilize composite structure and enhance mechanical properties | Concentration affects porosity and functional group availability |
| Lignin | Kraft or Organosolv lignin (≥97% purity) | Bio-based filler enhancing functionality and sustainability | Improves thermal stability and provides additional phenolic groups |
| Polyethylenimine (PEI) | Branched, 50% aqueous solution | Surface amination for enhanced metal binding | Increases density of amino groups for improved cation adsorption |
| pH Adjusters | NaOH, HCl (0.1-1M solutions) | Optimize synthesis conditions and adsorption pH | Critical for protonation of amino groups and precipitation reactions |
| Model Pollutants | K₂Cr₂O₇, Cu(NO₃)₂, Pb(NO₃)₂, Reactive Orange 16 | Standardized contaminants for performance evaluation | Enable comparative assessment across different studies |
Advanced characterization represents another essential component of the research toolkit. Fourier-Transform Infrared Spectroscopy (FTIR) identifies functional groups and their involvement in adsorption [29] [80]. Scanning Electron Microscopy (SEM) reveals surface morphology and composite structure [29] [80]. X-ray Diffraction (XRD) analyzes crystallinity and phase composition of magnetic nanoparticles [29]. Surface Area Analyzers (BET method) quantify specific surface area and pore size distribution, critical for understanding adsorption capacity [29] [47].
Nanomagnetic chitosan composites represent a promising class of adsorbents that effectively balance removal efficiency with economic viability and environmental sustainability. The comparative analysis presented demonstrates that these materials achieve competitive adsorption capacities (ranging from 50-400 mg/g depending on composition and target pollutant) while leveraging renewable resources and enabling efficient recovery through magnetic separation [34] [29] [47].
The integration of eco-design principles throughout composite development - from utilizing waste-derived materials like chitosan and lignin to optimizing synthesis protocols for minimal environmental impact - aligns with broader sustainability goals in materials science [81] [82]. The case study of Rhoades Hall renovation demonstrates how lifecycle thinking and sustainable design principles deliver significant economic and environmental returns [81], providing a valuable framework for advancing nanomagnetic chitosan composites.
Future research should address several key challenges to enable widespread implementation: standardized testing protocols for reliable performance comparison, pilot-scale validation under real-world conditions, comprehensive lifecycle assessments to quantify environmental benefits, and optimized regeneration protocols to enhance economic viability. Additionally, exploring novel functionalization strategies to improve selectivity for specific contaminants and developing multifunctional composites capable of simultaneous removal of diverse pollutant classes represent promising research directions.
As water pollution concerns continue to grow globally, nanomagnetic chitosan composites offer a sustainable solution pathway that aligns with circular economy principles while delivering the performance required for effective water treatment applications. Their development exemplifies how interdisciplinary approaches combining materials science, environmental engineering, and green chemistry can address pressing environmental challenges through technologically advanced yet ecologically responsible solutions.
Benchmarking and Validation: From Laboratory Data to Practical Application
The contamination of water resources by heavy metals poses a significant threat to global ecosystems and human health. In response, the development of advanced adsorbent materials has become a critical research focus, with nanocomposites emerging as particularly promising solutions. Among these, chitosan-based composites have garnered substantial scientific interest due to their biodegradability, abundance of functional groups, and environmental compatibility [29] [34].
This guide provides a systematic comparison of the heavy metal removal efficiency of various nanocomposites, with particular emphasis on nanomagnetic chitosan-based materials. By synthesizing experimental data from recent studies, we offer researchers a comprehensive reference for selecting and developing adsorbents tailored to specific heavy metal contaminants. The performance metrics, experimental protocols, and mechanistic insights presented herein aim to facilitate advancements in water purification technologies and support the work of researchers, scientists, and environmental professionals engaged in pollution remediation.
Performance Comparison of Composite Adsorbents
The adsorption capacity of a material is a primary indicator of its effectiveness in removing heavy metals from aqueous solutions. This section provides comparative performance data for various composite adsorbents documented in recent scientific literature.
Table 1: Adsorption Performance of Chitosan-Based Composites for Heavy Metal Removal
| Composite Material | Target Heavy Metal | Maximum Adsorption Capacity (mg/g) | Optimal pH | Reference |
|---|---|---|---|---|
| Magnetic Chitosan Composite (MCC) | Pb(II) | 220.9 | ~8-8.5 | [5] [6] |
| Magnetic Chitosan Composite (MCC) | Cu(II) | 216.8 | ~8-8.5 | [5] [6] |
| Magnetic Chitosan Composite (MCC) | Ni(II) | 108.9 | ~8-8.5 | [5] [6] |
| Chitosan + 50% Lignin Biocomposite | Cr(VI) | 72.61 | 2 | [29] |
| Chitosan + 50% Lignin Biocomposite | RO16 Dye | 79.76 | 6 | [29] |
| Magnetic Chitosan/Sludge Biochar | Cu(II) | 55.16 | 5 | [83] |
| Amino-thiourea Modified Chitosan-Magnetic Biochar (TMBC) | Cd(II) | 137.3 (at 318 K) | Not Specified | [83] |
| Chitosan-EDTA Functionalized Magnetic Biochar (E-CMBC) | Pb(II) | 156.68 | 3.0 | [83] |
Table 2: Performance of Other Adsorbent Materials for Heavy Metal Removal
| Adsorbent Material | Target Heavy Metal | Maximum Adsorption Capacity (mg/g) | Optimal pH | Reference |
|---|---|---|---|---|
| Bone Char | Cr(VI) | ~100% removal at 10 mg/L | 1 | [14] |
| Schwertmannite | As(V), Cr(VI) | Varies with synthesis method | Contingent on specific metal | [84] |
| Activated Carbon | Various Heavy Metals | 75-96% removal efficiency | Varies | [85] |
The data reveal significant variations in adsorption capacities across different composite materials, influenced by factors including surface functionality, structural properties, and experimental conditions. Magnetic chitosan composites demonstrate particularly high efficacy, especially for lead and copper ions [5] [6]. The performance of chitosan-lignin biocomposites against both dye and chromium contamination highlights their versatility [29], while modified biochar composites show enhanced capacity for specific metals like cadmium and lead [83].
Experimental Protocols and Methodologies
Understanding the experimental procedures used to generate performance data is crucial for interpretation and replication. This section details common methodologies for adsorbent preparation and evaluation.
Composite Synthesis Protocols
Preparation of Chitosan-Lignin Biocomposite: The synthesis involves dissolving chitosan in 0.1 mol/L acetic acid with continuous stirring for 24 hours. The solution is filtered, degassed, and cast onto glass substrates to form films. Separately, lignin is dissolved in double-distilled water. The lignin solution is gradually added to the chitosan solution and stirred at 300 rpm for three hours. The resulting biocomposite is filtered, air-dried for 48 hours, ground into powder, washed with distilled water, vacuum filtered, and finally dried at 100°C for three hours [29].
Synthesis of Magnetic Chitosan/Sludge Biochar Composite: Sludge biochar is first prepared by pyrolyzing dried sewage sludge through gradual heating in a muffle furnace. The magnetic chitosan composite is then prepared using chitosan, Fe₃O₄, and the prepared sludge biochar as raw materials. The resulting composite enables rapid solid-liquid separation under an applied magnetic field [83].
Production of Bone Char: Animal bones are cleaned with distilled water and dried to eliminate moisture and organic matter. Pyrolysis is conducted at controlled temperatures between 400°C and 600°C in an oxygen-deficient environment. Post-treatment may include washing with acid to enhance surface area and functional groups [14].
Adsorption Experiment Methodology
Standard batch adsorption experiments typically involve the following steps:
- Solution Preparation: Stock solutions (e.g., 500 mg/L) of target heavy metals are prepared and diluted to desired concentrations (10-100 mg/L) [29].
- Parameter Optimization: Experiments investigate the effects of adsorbent dosage (e.g., 0.05 g), initial metal concentration, solution pH (adjusted using NaOH or HCl), temperature (25-45°C), and contact time (e.g., 180 minutes) [29] [83].
- Agitation and Sampling: The mixture is stirred at constant speed (e.g., 240 rpm), with samples collected at regular intervals [29].
- Analysis: Samples are centrifuged, and supernatant concentrations are analyzed using techniques like UV-Vis spectrophotometry [29].
The workflow for the synthesis and evaluation of composite adsorbents for heavy metals can be visualized as follows:
Adsorption Mechanisms and Kinetic Studies
Understanding the underlying mechanisms of heavy metal adsorption is essential for material optimization and application.
Primary Adsorption Mechanisms
The removal of heavy metals by composite adsorbents occurs through several interconnected mechanisms:
- Electrostatic Attraction: Protonated amino and hydroxyl groups on chitosan interact with anionic metal complexes [29].
- Chemical Complexation: Functional groups (amino, hydroxyl) form coordination bonds with metal ions [5] [6].
- Ion Exchange: Metal ions replace cations bound to the adsorbent surface [35].
- Physical Adsorption: Weak van der Waals forces contribute to metal ion attachment [35].
- Chemical Precipitation: Metal ions transform into insoluble precipitates under specific pH conditions [35].
Kinetic and Isotherm Modeling
Most adsorption studies employ kinetic and isotherm models to understand the adsorption process:
- Pseudo-First-Order Kinetics: Describes physical adsorption processes [83].
- Pseudo-Second-Order Kinetics: Indicates chemisorption as the rate-limiting step [83]; commonly reported for chitosan composites [83] [14].
- Langmuir Isotherm: Suggests monolayer coverage on a homogeneous surface [83] [14].
- Freundlich Isotherm: Indicates multilayer adsorption on heterogeneous surfaces [14].
For chitosan-lignin biocomposites, advanced statistical physics modeling (heterogeneous monolayer model with two functional groups) has revealed that reactive orange 16 dye and Cr(VI) interact with two distinct functional groups, operating through a multi-ionic mechanism with significant aggregation at specific temperatures [29].
The following diagram illustrates the primary mechanisms involved in heavy metal adsorption by chitosan-based composites:
The Scientist's Toolkit: Essential Research Reagents and Materials
This section details key reagents and materials commonly employed in heavy metal adsorption research, providing researchers with a practical reference for experimental design.
Table 3: Essential Research Reagents and Materials for Adsorption Studies
| Reagent/Material | Function/Application | Examples/Specific Uses |
|---|---|---|
| Chitosan | Primary adsorbent matrix | Base material for composite formation; provides amino and hydroxyl functional groups [29] [83] |
| Lignin | Biocomposite component | Enhances surface functionality and adsorption efficiency in chitosan composites [29] |
| Fe₃O4 (Magnetite) | Magnetic component | Enables magnetic separation of adsorbent from treated water [5] [83] [6] |
| Sludge Biochar | Porous adsorbent substrate | Provides high surface area and porosity; waste valorization application [83] |
| Acetic Acid | Solvent for chitosan | Used to dissolve chitosan prior to composite formation [29] |
| NaOH/HCl | pH adjustment | Critical for optimizing adsorption conditions for specific heavy metals [29] |
| Potassium Dichromate | Cr(VI) source | Standard compound for preparing chromium solutions [29] |
| 1,5-diphenylcarbazide | Complexing agent for Cr(VI) analysis | Forms colored complex for spectrophotometric detection of chromium [29] |
| Bone Char | Alternative adsorbent | Effective for Cr(VI) removal under highly acidic conditions [14] |
| Activated Carbon | Reference adsorbent | Benchmark material for comparison studies [85] |
This comparative analysis demonstrates that nanomagnetic chitosan composites represent a highly effective class of adsorbents for heavy metal removal from aqueous solutions. The performance data reveal that material composition significantly influences adsorption capacity, with modified chitosan composites consistently outperforming conventional adsorbents for multiple heavy metal contaminants.
The experimental protocols and mechanistic insights provided herein offer researchers a robust foundation for material selection, experimental design, and result interpretation. Future research directions should focus on optimizing synthesis parameters for enhanced capacity and selectivity, improving regeneration capabilities for multiple adsorption-desorption cycles, and validating performance at pilot scales under real-world conditions.
As water pollution continues to pose global challenges, the development and refinement of advanced composite adsorbents will play an increasingly vital role in ensuring water security and environmental sustainability.
The contamination of water resources by heavy metals represents a significant global environmental challenge, necessitating the development of efficient remediation technologies [48]. Among various water treatment approaches, adsorption has gained widespread acceptance due to its high efficiency, simple operation, and cost-effectiveness [48] [86]. In recent years, nanomagnetic chitosan composites have emerged as frontier adsorbents that combine the excellent metal-binding capacity of chitosan with the facile separation capability of magnetic nanoparticles [34] [47]. Chitosan, a biodegradable cationic polysaccharide derived from chitin, possesses abundant amino and hydroxyl functional groups that enable multiple interaction mechanisms with metal ions [48]. When combined with magnetic components such as Fe₃O₄ or nickel ferrite, these composites can be efficiently separated from treated solutions using an external magnetic field, addressing a critical challenge in nanoparticle-based water treatment [86] [47]. This review provides a comprehensive comparative analysis of different nanomagnetic chitosan composites, with a specific focus on elucidating the fundamental mechanisms—electrostatic interaction, chelation, and ion exchange—that govern their efficacy in heavy metal removal.
Fundamental Mechanisms of Metal Removal
The adsorption performance of nanomagnetic chitosan composites stems from the synergistic operation of multiple mechanisms that simultaneously contribute to metal ion sequestration.
Electrostatic Interaction
Electrostatic attraction occurs between positively charged amino groups (-NH₃⁺) on the chitosan backbone and anionic metal species in solution [48] [86]. This mechanism is particularly significant for metals such as chromium, which primarily exists as oxyanions (HCrO₄⁻, CrO₄²⁻) in aqueous environments [86]. The protonation of amino groups is pH-dependent, with increased protonation occurring under acidic conditions, thereby enhancing the electrostatic attraction of anionic metal species [86] [29]. For instance, in the case of Cr(VI) removal by polyaniline@magnetic chitosan (PANI@MCTS), the protonated amino groups effectively attracted chromate anions, facilitating subsequent reduction to less toxic Cr(III) [86].
Chelation
Chitosan contains abundant amino and hydroxyl groups that can form coordinated bonds with metal cations through donation of electron pairs, resulting in stable chelate complexes [48] [87]. This mechanism is particularly effective for cations such as Cu(II), Pb(II), Cd(II), and rare earth elements [87] [13]. The chelation capacity is significantly higher in chitosan compared to chitin due to the greater availability of amino groups after deacetylation [48]. Functionalization with specific ligands containing donor atoms (e.g., nitrogen, oxygen, sulfur) further enhances the chelation potential. For example, cysteine-functionalized magnetic chitosan nanoparticles demonstrated exceptional chelating capability for rare earth metals (La(III), Nd(III), and Yb(III)) through coordination with sulfur and nitrogen atoms [87].
Ion Exchange
The ion exchange mechanism involves the replacement of ions associated with the chitosan composite (such as H⁺ or Na⁺) with target metal ions from solution [48] [87]. This process is influenced by the relative affinity of metal ions for the functional groups on the adsorbent surface and their concentrations in solution [87]. In cysteine-functionalized magnetic chitosan, cationic rare earth metal species were adsorbed through a combination of chelation and anion-exchange mechanisms [87]. The ion exchange capacity can be modulated through chemical modifications that introduce additional ionic functional groups to the chitosan structure.
Table 1: Primary Mechanisms for Various Heavy Metals
| Heavy Metal | Primary Removal Mechanism | Composite Example | Supporting Evidence |
|---|---|---|---|
| Cr(VI) | Electrostatic attraction followed by reduction | PANI@MCTS [86] | Protonated amino groups attract chromate anions; XPS confirmation of Cr(III) formation |
| Pb(II), Cu(II), Cd(II) | Chelation | TPP/V-CMN [13] | Coordination with amino and hydroxyl groups; pH-dependent uptake |
| Rare Earth Elements (La, Nd, Yb) | Chelation & Ion Exchange | Cysteine-functionalized CS [87] | Coordination with S/N atoms; Langmuir isotherm fit |
| Mixed Systems (RO16 dye + Cr(VI)) | Multi-mechanism | Chitosan-lignin [29] | Statistical physics modeling identified two distinct functional group interactions |
Comparative Analysis of Nanomagnetic Chitosan Composites
Synthesis and Modification Strategies
Nanomagnetic chitosan composites are primarily synthesized through co-precipitation methods, where magnetic nanoparticles are formed in the presence of chitosan [47] [13]. Alternative approaches include cross-linking, spray drying, and electrostatic dropping [47]. Surface modification plays a crucial role in enhancing adsorption performance and introducing specific functionalities:
- Polymer Grafting: Polyaniline grafting onto magnetic chitosan (PANI@MCTS) introduces additional nitrogen-containing functional groups that enhance both adsorption capacity and redox activity for Cr(VI) removal [86].
- Cross-linking: Cross-linking with agents such as epichlorohydrin improves chemical stability but may reduce adsorption capacity by occupying functional groups [87].
- Ligand Functionalization: Incorporating specific ligands like cysteine provides additional chelating sites through sulfur, nitrogen, and oxygen atoms, enabling selective metal recovery [87].
Table 2: Comparison of Nanomagnetic Chitosan Composites
| Composite Type | Maximum Adsorption Capacity (mg/g) | Target Pollutants | Key Advantages | Limitations |
|---|---|---|---|---|
| PANI@MCTS [86] | 186.6 | Cr(VI) | Simultaneous adsorption & reduction; fast kinetics (15 min) | Specific to redox-active metals |
| Cs-NiFe₂O₄ [43] | - | Antibacterial applications | Enhanced antibacterial & antioxidant properties | Limited metal adsorption data |
| Cysteine-functionalized [87] | ~45-55 (for REEs) | La(III), Nd(III), Yb(III) | Selective for heavy REEs; efficient regeneration | Moderate capacity for light REEs |
| TPP-CMN [13] | 87.25-99.96 | Cd(II), Co(II), Cu(II), Pb(II) | High multi-metal capacity; rapid equilibrium (15 min) | Lower surface area (8.75 m²/g) |
| V-CMN [13] | 88.75-99.89 | Cd(II), Co(II), Cu(II), Pb(II) | High multi-metal capacity; good reusability | Lower surface area (6.96 m²/g) |
| Chitosan-lignin [29] | 59.43-79.76 (RO16), 52.06-72.61 (Cr(VI)) | RO16 dye, Cr(VI) | Effective for mixed pollutants; biodegradable | Lower capacity than specialized composites |
Structural and Magnetic Properties
The structural characteristics of nanomagnetic chitosan composites significantly influence their adsorption performance. Typical saturation magnetization values range from 7.2 to 17.3 emu/g for coated nanoparticles, sufficient for effective magnetic separation [43] [13]. The incorporation of magnetic components (e.g., Fe₃O₄, NiFe₂O₄) creates composites with superparamagnetic properties, preventing permanent magnetization and ensuring redispersion after magnetic separation [43] [47]. Specific surface area varies considerably among composites, with modified chitosan materials typically exhibiting values between 6.96-8.75 m²/g [13], while more porous architectures can achieve significantly higher surface areas. Particle size distribution analysis reveals that most nanomagnetic chitosan composites fall within the 10-40 nm range [43] [87], providing high surface-to-volume ratios that enhance adsorption kinetics and capacity.
Experimental Protocols and Methodologies
Synthesis of Chitosan-Coated Magnetic Nanoparticles
Chitosan-Coated Magnetic Nanoparticle Synthesis
The co-precipitation method represents the most widely employed synthesis approach [47] [13]. A typical protocol involves:
- Chitosan Solution Preparation: Dissolve chitosan powder (1-3% w/v) in dilute acetic acid solution (1-2% v/v) with continuous stirring until complete dissolution [13].
- Magnetic Nanoparticle Incorporation:
- In-situ method: Add Fe²⁺ and Fe³⁺ salts (molar ratio 1:2) directly to the chitosan solution, then adjust pH to 10-12 with NaOH or NH₄OH to precipitate magnetic nanoparticles within the chitosan matrix [47].
- Two-step method: Add pre-synthesized magnetic nanoparticles (e.g., Fe₃O₄) to the chitosan solution and disperse via ultrasonication for 20-30 minutes [13].
- Cross-linking: Add cross-linking agents such as glutaraldehyde or epichlorohydrin to improve chemical stability [87].
- Product Recovery: Separate the resulting composite by filtration or magnetic separation, wash with ethanol and water, and dry at 50-60°C [13].
Batch Adsorption Experiments
Batch Adsorption Experimental Workflow
Standardized batch adsorption experiments are crucial for evaluating composite performance [13] [29]:
- Solution Preparation: Prepare stock solutions of target metals (e.g., Cd(II), Co(II), Cu(II), Pb(II), Cr(VI)) at 1000 mg/L and dilute to desired concentrations (10-500 mg/L) [13].
- pH Adjustment: Adjust solution pH using 0.1 M NaOH or HCl to optimize adsorption conditions (typically pH 2-6 depending on target metal) [29].
- Adsorption Process: Add a known mass of nanomagnetic chitosan composite (0.01-0.1 g) to the metal solution and agitate at constant temperature (25-45°C) for predetermined time intervals (15 minutes to 24 hours) [13] [29].
- Separation: Separate the adsorbent using an external magnet [86] [47].
- Analysis: Measure residual metal concentration in supernatant using atomic absorption spectroscopy (AAS), UV-Vis spectrophotometry, or inductively coupled plasma techniques [13] [29].
- Capacity Calculation: Calculate adsorption capacity using the formula: [ qe = \frac{(C0 - Ce) \times V}{m} ] where ( qe ) is adsorption capacity (mg/g), ( C0 ) and ( Ce ) are initial and equilibrium concentrations (mg/L), ( V ) is solution volume (L), and ( m ) is adsorbent mass (g) [13].
Analytical Characterization Techniques
Comprehensive characterization is essential for understanding structure-property relationships:
- FTIR Spectroscopy: Identifies functional groups and their involvement in metal binding through shifts in characteristic peaks (amine ~1590 cm⁻¹, hydroxyl ~3400 cm⁻¹) [43] [87].
- XRD Analysis: Determines crystallinity and successful incorporation of magnetic phases (characteristic peaks for Fe₃O₄ at 2θ = 30.1°, 35.5°, 43.1°, 57.0°, 62.6°) [43] [13].
- SEM/TEM Microscopy: Reveals surface morphology, particle size distribution, and elemental composition [43] [13].
- VSM Analysis: Measures magnetic properties including saturation magnetization, coercivity, and remanence [43] [13].
- XPS Spectroscopy: Provides information on elemental composition, chemical states, and specific metal-adsorbent interactions [86].
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 3: Essential Research Reagents and Materials
| Reagent/Material | Function/Application | Examples/Specifications |
|---|---|---|
| Chitosan | Primary biopolymer matrix providing functional groups | Low molecular weight (50,000-190,000 Da); Degree of deacetylation ≥85% [43] [13] |
| Iron Salts | Magnetic nanoparticle precursor | FeCl₃·6H₂O, FeSO₄·7H₂O (analytical grade) [86] [13] |
| Nickel Ferrite | Alternative magnetic component | NiFe₂O₄ for enhanced magnetic properties [43] |
| Cross-linking Agents | Improve chemical stability | Glutaraldehyde, epichlorohydrin, tripolyphosphate (TPP) [87] [13] |
| Functionalization Ligands | Enhance selectivity and capacity | Cysteine, polyaniline, vanillin [86] [87] [13] |
| pH Adjustment Reagents | Control solution pH for optimization | NaOH, HCl (0.1-1.0 M solutions) [13] [29] |
| Metal Salts | Prepare standard solutions for testing | CdSO₄, CoCl₂, CuSO₄, Pb(NO₃)₂, K₂Cr₂O₇ (analytical grade) [13] [29] |
Performance Comparison and Mechanistic Insights
Quantitative Performance Metrics
The adsorption performance of nanomagnetic chitosan composites varies significantly based on their specific composition, modification strategies, and target metals:
- TPP-CMN and V-CMN demonstrate exceptional capacities for multiple heavy metals, with Pb(II) removal reaching nearly 100 mg/g and rapid equilibrium within 15-30 minutes [13].
- PANI@MCTS exhibits specialized performance for Cr(VI) with a capacity of 186.6 mg/g, leveraging both adsorption and reduction mechanisms [86].
- Cysteine-functionalized composites show selective recovery of heavy rare earth elements (Yb(III)) over light counterparts (La(III), Nd(III)) [87].
- Chitosan-lignin biocomposites provide balanced performance for mixed pollutant systems, simultaneously removing dyes (RO16) and Cr(VI) with capacities of 59.43-79.76 mg/g and 52.06-72.61 mg/g, respectively [29].
Mechanistic Pathways for Different Composites
Heavy Metal Removal Mechanisms
The predominance of specific mechanisms depends on both adsorbent characteristics and metal solution chemistry:
For Cationic Metals (Pb²⁺, Cu²⁺, Cd²⁺): Chelation represents the primary mechanism, where metal ions form coordination complexes with electron-donating amino and hydroxyl groups on chitosan [48] [13]. The efficiency follows the general order: Pb(II) > Cu(II) > Cd(II) > Co(II), consistent with the Irving-Williams series of metal complex stability [13].
For Oxyanionic Metals (Cr(VI)): Electrostatic attraction initially brings anionic chromate species to the protonated amino groups, followed by reduction to Cr(III) when redox-active components like polyaniline are present [86]. The reduction step is confirmed by XPS analysis showing both Cr(VI) and Cr(III) on the adsorbent surface [86].
For Rare Earth Elements: A combination of chelation and ion exchange mechanisms operates, with heavier rare earths (Yb(III)) exhibiting greater affinity due to lanthanide contraction effects that result in higher charge density [87].
In Multi-component Systems: Competitive adsorption occurs where metals with higher affinity can displace those with lower affinity, though some composites exhibit selective binding based on tailored functionalization [87] [29].
Nanomagnetic chitosan composites represent a versatile class of adsorbents with demonstrated efficacy for heavy metal removal from aqueous solutions. Their performance stems from the synergistic operation of electrostatic interactions, chelation, and ion exchange mechanisms, with the relative contribution of each mechanism dependent on composite design and target metals. Comparative analysis reveals that functionalization strategies significantly enhance both capacity and selectivity, with composites like PANI@MCTS and cysteine-functionalized materials exhibiting specialized performance for specific metal groups. The incorporation of magnetic components enables facile separation, addressing a critical challenge in nanoparticle applications. Future research should focus on developing multifunctional composites capable of simultaneous removal of multiple pollutant types, optimizing regeneration protocols for economic viability, and scaling up synthesis procedures for industrial implementation. The mechanistic understanding presented in this review provides a foundation for rational design of next-generation nanomagnetic chitosan composites with enhanced performance characteristics for sustainable water treatment applications.
The evaluation of adsorbent materials for heavy metal removal has traditionally relied heavily on batch studies, which provide essential preliminary data on adsorption capacity and kinetics. However, the transferability of these batch-derived parameters to continuous-flow column systems—the configuration most relevant to industrial application—remains a critical challenge in environmental remediation research. While batch experiments offer valuable insights into equilibrium behavior and sorption mechanisms under controlled conditions, they often fail to accurately predict performance in dynamic column systems where factors such as flow rate, bed height, and mass transfer zones significantly influence overall efficiency [88]. This comparative guide objectively examines the performance of various nanomagnetic chitosan composites across both experimental paradigms, with particular emphasis on breakthrough curve modeling as an essential validation tool for predicting real-world applicability in water treatment systems.
The fundamental limitation of batch studies lies in their static nature, which does not represent the continuous flow conditions of industrial wastewater treatment. As Pathirana et al. (2022) demonstrated, the translation of batch data to column performance is not always straightforward, with the initial sorption rate from batch experiments being one of the few parameters that can reasonably predict breakthrough time in column systems [88]. This validation gap necessitates rigorous column adsorption studies coupled with appropriate mathematical modeling to accurately assess adsorbent performance under dynamic conditions relevant to practical applications.
Comparative Performance of Nanomagnetic Chitosan Composites
Adsorption Capacity Across Experimental Systems
Table 1: Comparison of Heavy Metal Removal Performance in Batch vs. Column Systems
| Adsorbent Material | Target Contaminant | Maximum Batch Adsorption Capacity (mg/g) | Maximum Column Adsorption Capacity (mg/g) | Optimal pH | Reference |
|---|---|---|---|---|---|
| Magnetic chitosan nanohybrid (r-MCS) | Cu(II) | 0.99 mmol/g (∼63 mg/g) | Not reported | 5.0 | [89] |
| Amine-functionalized magnetic chitosan (TA-type) | Cu(II) | 3.29 mmol/g (∼209 mg/g) | Not reported | 5.0 | [89] |
| Amino acid-functionalized magnetic chitosan (C-type) | Cu(II) | 1.92 mmol/g (∼122 mg/g) | Not reported | 5.0 | [89] |
| Coal fly ash-chitosan composite (MFA-CS) | Pb(II) | 352.19 | 255.61 | Optimized via RSM | [90] |
| Coal fly ash-chitosan composite (MFA-CS) | Cr(VI) | 265.13 | 42.08 | Optimized via RSM | [90] |
| Magnetic chitosan composite (MCC) | Pb(II) | 220.9 | Not reported | Not specified | [5] |
| Magnetic chitosan composite (MCC) | Cu(II) | 216.8 | Not reported | Not specified | [5] |
| Magnetic chitosan composite (MCC) | Ni(II) | 108.9 | Not reported | Not specified | [5] |
The comparative data reveals significant disparities between batch and column adsorption capacities for the same adsorbent materials. The coal fly ash-chitosan composite (MFA-CS) demonstrates a particularly notable decrease in capacity for Cr(VI) from 265.13 mg/g in batch systems to 42.08 mg/g in column studies, highlighting the critical importance of validation beyond batch experiments [90]. This reduction in performance under continuous flow conditions may be attributed to mass transfer limitations, insufficient contact time, or channeling effects within the packed bed.
Functionalization strategies significantly enhance adsorption performance, as evidenced by the superior capacity of amine-functionalized magnetic chitosan (TA-type) for copper removal (209 mg/g) compared to its unfunctionalized counterpart (63 mg/g) [89]. However, it is noteworthy that most studies on functionalized nanomagnetic chitosan composites lack corresponding column data, representing a significant research gap in the validation pipeline for these advanced materials.
Breakthrough Behavior and Dynamic Performance
Table 2: Breakthrough Curve Modeling Parameters for Chitosan-Based Adsorbents
| Adsorbent Material | Target Contaminant | Optimal Bed Height (mm) | Optimal Flow Rate (mL/min) | Best-Fit Model | R² Value | Reference |
|---|---|---|---|---|---|---|
| Coconut-based PACC | Pb(II) | 150 | 4 | Thomas & Yoon-Nelson | ≥0.96 | [91] |
| Coal fly ash-chitosan composite | Pb(II) | Not specified | Not specified | Thomas | Not reported | [90] |
| Coal fly ash-chitosan composite | Cr(VI) | Not specified | Not specified | Thomas | Not reported | [90] |
Breakthrough curve modeling provides critical insights for system design and scalability. The Thomas and Yoon-Nelson models have demonstrated exceptional efficacy in describing the dynamic adsorption behavior of chitosan-based composites in fixed-bed columns, with R² values ≥0.96 observed for lead removal using coconut-based polyurethane-activated carbon composite (PACC) [91]. These models effectively predict breakthrough times and adsorption capacities under varying operational parameters, offering valuable tools for designing full-scale treatment systems.
Operational parameters significantly influence column performance. Increased bed height typically enhances adsorption efficiency by providing more binding sites and increasing hydraulic retention time, while lower flow rates improve removal efficiency by allowing sufficient contact time between adsorbate and adsorbent [91]. The coconut-based PACC exhibited optimal lead removal at 150 mm bed height and 4 mL/min flow rate, highlighting the importance of parameter optimization for each specific adsorbent-adsorbate system.
Experimental Protocols and Methodologies
Column Adsorption Experimental Design
Figure 1: Fixed-Bed Column Experimental Workflow for Adsorption Studies
Fixed-bed column experiments follow a systematic approach to evaluate adsorbent performance under dynamic conditions. The standard methodology involves packing a glass column with a predetermined amount of adsorbent to achieve specific bed heights (typically 50-150 mm) [91]. The contaminant solution is then pumped through the column in down-flow mode using a peristaltic pump, allowing precise control over flow rates (generally 4-8 mL/min) [91]. Effluent samples are collected at regular time intervals and analyzed for residual contaminant concentration using appropriate analytical techniques such as atomic absorption spectroscopy (AAS) or inductively coupled plasma optical emission spectrometry (ICP-OES).
Critical operational parameters requiring optimization include:
- Bed height: Affects the number of available binding sites and residence time
- Flow rate: Influences contact time between adsorbate and adsorbent
- Influent pH: Impacts metal speciation and adsorbent surface charge
- Initial contaminant concentration: Affects the driving force for adsorption
The experiment continues until the effluent concentration reaches 90-95% of the influent concentration (C/C₀ = 0.90-0.95), indicating column exhaustion [88]. The resulting breakthrough curve, which plots C/C₀ against time or throughput volume, provides essential data for modeling and scale-up calculations.
Breakthrough Curve Modeling Approaches
Figure 2: Breakthrough Curve Modeling Methodology for System Design
Three primary mathematical models are commonly employed to analyze fixed-bed column data and predict scalable system performance:
Thomas Model: This widely used model assumes pseudo-second-order kinetics and negligible axial dispersion. It effectively predicts the adsorption capacity of the bed and the Thomas rate constant using the following equation:
[ \frac{Ct}{C0} = \frac{1}{1 + \exp\left(\frac{k{Th}q0m}{Q} - k{Th}C0t\right)} ]
Where Cₜ is the effluent concentration at time t, C₀ is the influent concentration, k({}_{\text{Th}}) is the Thomas rate constant (mL/min·mg), q₀ is the equilibrium adsorption capacity (mg/g), m is the mass of adsorbent (g), and Q is the flow rate (mL/min) [91].
Yoon-Nelson Model: This simpler model requires no specific details about the adsorbate or adsorbent characteristics and predicts the time required for 50% adsorbate breakthrough:
[ \frac{Ct}{C0 - Ct} = \exp\left(k{YN}\tau - k_{YN}t\right) ]
Where k({}_{\text{YN}}) is the Yoon-Nelson rate constant (min⁻¹), and τ is the time required for 50% breakthrough (min) [91].
Bohart-Adams Model: Primarily used for describing the initial part of the breakthrough curve, this model focuses on surface reaction theory and is useful for predicting initial adsorption rates:
[ \ln\left(\frac{Ct}{C0}\right) = k{AB}C0t - k{AB}N0\frac{Z}{U_0} ]
Where k({}_{\text{AB}}) is the Bohart-Adams rate constant (L/mg·min), N₀ is the saturation concentration (mg/L), Z is the bed depth (cm), and U₀ is the superficial velocity (cm/min) [91].
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 3: Essential Research Reagents and Materials for Adsorption Studies
| Reagent/Material | Function/Application | Specification Considerations |
|---|---|---|
| Chitosan | Primary adsorbent matrix | Low molecular weight preferred for improved gelation and permeability [68] |
| Ferric chloride (FeCl₃) | Magnetite precursor for magnetic functionalization | Anhydrous form for consistent iron content [89] |
| Ferrous chloride (FeCl₂·4H₂O) | Magnetite co-precursor | Maintain 2:1 Fe³⁺:Fe²⁺ molar ratio for stoichiometric magnetite formation [89] |
| Sodium tripolyphosphate (TPP) | Ionic crosslinking agent | 5M solution for effective bead formation [68] |
| Epichlorohydrin | Covalent crosslinking agent | Alkaline conditions (pH 10) required for reaction with chitosan [89] |
| Diethylenetriamine (DETA) | Amine functionalization | Enhances copper binding capacity through chelation [89] |
| Amino acids (cysteine, alanine, serine) | Surface functionalization | Cysteine particularly effective due to thiol group metal affinity [89] |
| Heavy metal salts (Pb(NO₃)₂, K₂Cr₂O₇, Cu(NO₃)₂) | Adsorbate solutions | Analytical grade for precise concentration preparation [91] [90] |
| Nitric acid (HNO₃) | Desorption/regeneration agent | 0.1-0.2M concentration for effective metal recovery [90] |
| Sodium hydroxide (NaOH) | pH adjustment | 0.1M for neutralization and precipitation [68] |
The selection of appropriate reagents and materials significantly influences experimental outcomes in adsorption research. Chitosan quality is particularly critical, with low molecular weight variants preferred for their enhanced gelation properties and improved permeability when forming composite beads [68]. Similarly, precise control over precursor ratios in magnetic functionalization ensures consistent superparamagnetic properties, which facilitate adsorbent recovery using external magnetic fields [89].
Functionalization reagents should be selected based on their affinity for target contaminants. Diethylenetriamine and cysteine have demonstrated exceptional performance for copper removal, with amine-functionalized magnetic chitosan (TA-type) exhibiting approximately 3.3 times greater capacity than non-functionalized equivalents [89]. For regeneration studies, nitric acid (0.1-0.2M) has proven effective for multiple adsorption-desorption cycles, with some composites maintaining performance through 15 regeneration cycles [90].
The validation of nanomagnetic chitosan composites through column adsorption studies and breakthrough curve modeling represents a critical step in translating laboratory research to practical water treatment applications. While batch studies provide valuable preliminary data on adsorption mechanisms and capacity, they consistently overestimate performance compared to dynamic column systems. The significant disparity between batch and column capacities observed in materials like the coal fly ash-chitosan composite (265.13 mg/g vs. 42.08 mg/g for Cr(VI)) underscores the necessity of column validation [90].
The superior fit of Thomas and Yoon-Nelson models (R² ≥ 0.96) for describing breakthrough behavior provides researchers with reliable tools for predicting adsorbent performance and designing scaled-up systems [91]. Furthermore, functionalization strategies, particularly amine and amino acid modifications, demonstrate substantial improvements in adsorption capacity, though their performance in continuous-flow systems requires further investigation.
Future research should prioritize standardized column testing protocols for novel adsorbents, long-term stability assessments under continuous operation, and evaluation of multi-contaminant systems to better simulate real-world wastewater conditions. The integration of breakthrough curve modeling with economic analysis will further enhance the practical implementation of these promising materials in industrial wastewater treatment applications.
The treatment of industrial wastewater containing heavy metals represents a significant environmental challenge, necessitating the development of efficient, sustainable removal technologies. Among the various adsorbents being explored, nanomagnetic chitosan composites have emerged as particularly promising materials due to their high adsorption capacity, ease of magnetic separation, and environmental compatibility [47] [34]. This guide provides a comparative analysis of the performance of various nanomagnetic chitosan composites when applied to real wastewater samples and complex matrices, supporting informed material selection for researchers and wastewater treatment professionals.
Performance Comparison of Nanomagnetic Chitosan Composites
The efficacy of nanomagnetic chitosan composites is influenced by their synthesis method, functionalization, and the specific wastewater matrix. The table below summarizes the documented performance of various composites for heavy metal removal from different water sources.
Table 1: Performance comparison of nanomagnetic chitosan composites in wastewater treatment.
| Composite Type | Target Pollutant | Water Matrix | Key Performance Metrics | Reference |
|---|---|---|---|---|
| TPP/Vanillin-modified CMN [13] | Cd(II), Co(II), Cu(II), Pb(II) | Synthetic & Real Wastewater | Adsorption Capacity: 87-100 mg/g; Equilibrium Time: 15-30 min; Efficiency: Significant results on real wastewater. | [13] |
| Chitosan-coated Fe₃O₄ (CS/Fe₃O₄ NC) [18] | Heavy Metals (collective) | Petroleum Wastewater | Removal Efficiency: ~100% for heavy metals in 30 min; Oil Removal: 90%; TSS Reduction: 78%. | [18] |
| Magnetic Chitosan/Cellulose-Fe(III) [M-Ch/CNF-Fe(III)] [12] | Cr(VI), Cu(II), Pb(II) | Aqueous Solution | Effective elimination across varying pH, adsorbent doses, time, and temperature. Potential for industrial effluent application. | [12] |
| Chitosan-Lignin Biocomposite [29] | Cr(VI) | Aqueous Solution | Maximum Adsorbed Quantity: 52-73 mg/g. Mechanism identified as physisorption. | [29] |
| CS-based Magnetic Flocculants (MC, MCM, MCAA) [92] | Cr(III), Co(II), Pb(II) | Simulated Wastewater | Effective flocculation performance attributed to hydroxyl, carboxyl, and amino functional groups and magnetic properties. | [92] |
Experimental Protocols for Performance Assessment
A critical component of comparative analysis is the standardization of evaluation methods. The following protocols are commonly employed to assess the adsorption performance of nanomagnetic chitosan composites.
Composite Synthesis and Characterization
- Synthesis of Magnetic Core: The coprecipitation method is frequently used, involving the precipitation of Fe²⁺ and Fe³⁺ ions in an alkaline solution to form Fe₃O₄ nanoparticles [47] [13]. The sol-gel method is another alternative [12].
- Chitosan Coating and Functionalization: The magnetic nanoparticles are coated with chitosan, often dissolved in dilute acetic acid [47] [13]. Cross-linking agents like sodium tripolyphosphate (TPP) or functional modifiers like vanillin are used to enhance stability and adsorption properties [13].
- Characterization: Materials are characterized using Fourier-Transform Infrared Spectroscopy (FT-IR) to identify functional groups, Scanning Electron Microscopy (SEM) for morphology, X-ray Diffraction (XRD) for crystallinity, and Vibrating Sample Magnetometry (VSM) to confirm magnetic properties [12] [13] [92].
Batch Adsorption Experiments
Batch experiments are conducted to evaluate key performance parameters under controlled conditions [12] [13].
- Effect of pH: Experiments are performed across a pH range (e.g., 1.0-8.0) using solutions of target pollutants. The pH is adjusted with NaOH or HCl [12] [29].
- Effect of Adsorbent Dosage: Different amounts of the composite (e.g., 0.05-1.0 g) are added to a fixed volume and concentration of the pollutant solution [12].
- Adsorption Kinetics: Solutions are sampled at regular time intervals to determine the time required to reach adsorption equilibrium [13].
- Adsorption Isotherms: Experiments are conducted with varying initial pollutant concentrations at a constant temperature to model adsorption capacity [12].
Analysis and Efficiency Calculation
The concentration of heavy metals before and after adsorption is typically quantified using Atomic Absorption Spectroscopy (AAS) or UV-Vis Spectrophotometry [13]. The removal efficiency and adsorption capacity are then calculated.
Figure 1: A generalized workflow for evaluating the performance of nanomagnetic chitosan composites in wastewater treatment.
Mechanisms of Adsorption and Key Functional Groups
The high removal efficiency of nanomagnetic chitosan composites stems from the synergistic action of their components and multiple adsorption mechanisms.
- Magnetic Core (Fe₃O₄): Provides a high-surface-area substrate and enables rapid separation from treated water using an external magnet, preventing secondary pollution and facilitating adsorbent reuse [47] [18].
- Chitosan Biopolymer: Serves as the primary adsorption matrix. Its amino (-NH₂) and hydroxyl (-OH) groups are key to binding pollutants [93] [64]. In acidic conditions, the amino groups become protonated (-NH₃⁺), promoting electrostatic attraction with anionic metal species like Cr(VI) [29] [64].
- Enhanced Functionality: Cross-linking and modification introduce additional functional groups (e.g., carboxyl from citric acid), which enable other interactions such as chemical complexation (chelation), ion exchange, and physical adsorption [64] [13] [92].
The Scientist's Toolkit: Essential Research Reagents
The table below lists key reagents and materials essential for synthesizing and evaluating nanomagnetic chitosan composites.
Table 2: Essential research reagents and materials for working with nanomagnetic chitosan composites.
| Reagent/Material | Function/Application | Reference |
|---|---|---|
| Chitosan | Primary biopolymer for coating; provides amino and hydroxyl functional groups for metal binding. | [12] [13] |
| FeCl₃·6H₂O / FeSO₄·7H₂O | Iron precursors for the synthesis of magnetic Fe₃O₄ nanoparticles via coprecipitation. | [12] [13] |
| Sodium Tripolyphosphate (TPP) | Cross-linking agent used to enhance the chemical stability and mechanical strength of chitosan composites. | [13] |
| Vanillin | A functional modifier used to introduce aldehyde groups, potentially enhancing adsorption properties. | [13] |
| Acetic Acid | Solvent for dissolving chitosan to create a coating solution for magnetic nanoparticles. | [29] [13] |
| NaOH / NH₄OH | Used to create an alkaline environment necessary for the precipitation of Fe₃O₄ nanoparticles. | [12] [13] |
| Heavy Metal Salts | Model pollutants for adsorption experiments (e.g., K₂Cr₂O₇, CuSO₄·5H₂O, Pb(NO₃)₂). | [12] [29] [13] |
Nanomagnetic chitosan composites demonstrate robust performance in treating heavy metals in complex wastewater streams. Composites like TPP/Vanillin-modified CMN and Chitosan-coated Fe₃O₄ are particularly effective, showing high removal efficiencies and fast kinetics in real and simulated industrial wastewaters [18] [13]. The choice of optimal composite depends on the specific wastewater composition, target metals, and operational requirements. Future research should focus on scaling up synthesis, conducting long-term stability studies in real industrial settings, and further elucidating the adsorption mechanisms for complex multi-pollutant systems.
The removal of heavy metals from contaminated water is a critical environmental challenge. For decades, conventional technologies including activated carbon (AC) and ion exchange resins have been the cornerstone of industrial wastewater treatment. However, the emergence of novel adsorbents, particularly nanomagnetic chitosan composites, presents a paradigm shift. This guide provides an objective, data-driven comparison of these technologies, framing the performance of chitosan composites within the broader context of adsorptive water treatment. Designed for researchers and scientists, this analysis benchmarks the new against the conventional, using quantitative performance data and detailed experimental protocols to illuminate the comparative advantages.
Performance Benchmarking: Quantitative Data Comparison
The efficacy of any water treatment technology is ultimately quantified by its adsorption capacity, removal efficiency, and operational robustness. The following tables summarize key performance metrics for nanomagnetic chitosan composites alongside activated carbon and ion exchange resins.
Table 1: Comparison of Adsorption Capacities (mg/g) for Various Heavy Metals
| Adsorbent Material | Pb(II) | Cd(II) | Cu(II) | Cr(VI) | Co(II) |
|---|---|---|---|---|---|
| TPP-CMN (Magnetic Chitosan) [13] | 99.96 | 91.75 | 87.25 | - | 93.00 |
| V-CMN (Magnetic Chitosan) [13] | 99.89 | 92.50 | 88.75 | - | 94.00 |
| CS-KAC-Ag (Chitosan-Composite) [94] | - | 95.1%* | - | 91.7%* | 80.5%* |
| Chitosan/AC Composite [95] | - | 52.63 | - | - | - |
| BPAC@Al₂O₃@Chitosan [96] | 57.10 | 46.90 | - | - | - |
| Chitosan Strip [26] | - | - | - | 29.39%* | - |
| Chitosan-Magnetite Strip [26] | - | - | - | 92.33%* | - |
*Values marked with an asterisk denote percentage removal efficiency, not adsorption capacity in mg/g.
Table 2: Operational Advantages and Disadvantages Comparison
| Technology | Key Advantages | Key Disadvantages |
|---|---|---|
| Nanomagnetic Chitosan Composites | High adsorption capacity for multiple metals; Fast kinetics (e.g., 15-30 min equilibrium [13]); Easy magnetic separation; Reusability (≥5 cycles [94] [96]); Functionality over a broader pH range. | Mechanical strength and chemical stability can require cross-linking [97] [47]; Performance is pH-sensitive [92]. |
| Activated Carbon (AC) [97] [95] | High surface area; Well-established production and use. | Lower adsorption capacity for some metals compared to composites [95]; Less selective; Higher operational cost for regeneration. |
| Ion Exchange Resins [97] | Effective for cationic contaminants; High regeneration capacity. | Can be inefficient for heavy metals strongly chelated in complex solvents [97]; High operational and regeneration chemical cost [97]. |
Experimental Protocols for Key Studies
The performance data presented above are derived from rigorous experimental methodologies. Understanding these protocols is essential for a fair comparison and for replicating the results.
Synthesis of Nanomagnetic Chitosan Sorbents
A common and effective method for creating these composites is the two-step co-precipitation method [47].
- Step 1: Synthesis of Fe₃O₄ Magnetic Nanoparticles. This is typically achieved via chemical co-precipitation, where a mixture of ferrous (Fe²⁺) and ferric (Fe³⁺) ions in an aqueous solution is precipitated with a base like sodium hydroxide (NaOH) or ammonia under an inert atmosphere. The formation of black magnetite (Fe₃O₄) precipitates confirms the reaction. The nanoparticles are then washed with deionized water and ethanol [26] [47].
- Step 2: Coating with Chitosan. Chitosan is dissolved in a weak acetic acid solution. The pre-formed Fe₃O₄ nanoparticles are then added to this chitosan solution. The mixture is homogenized via vigorous stirring or sonication, often with the addition of a cross-linking agent like glutaraldehyde or sodium tripolyphosphate (TPP) to enhance the stability of the final composite. The resulting product is dried to obtain the magnetic chitosan nanocomposite [13] [47].
Batch Adsorption Experiments
The standard protocol for evaluating adsorption performance involves batch equilibrium studies [13].
- Preparation of Metal Solution: A stock solution of the target heavy metal ion (e.g., Cd²⁺, Pb²⁺) at a high concentration (e.g., 1000 mg/L) is prepared using a salt like cadmium chloride or lead nitrate. This is diluted to desired concentrations for experiments.
- pH Adjustment: The pH of the metal solution is adjusted using dilute NaOH or HCl. pH is a critical parameter as it affects the surface charge of the adsorbent and the speciation of the metal ions.
- Adsorption Process: A fixed dosage of the adsorbent is added to a known volume and concentration of the metal solution. The mixture is agitated in a shaker incubator at a constant temperature and speed for a predetermined time to reach equilibrium.
- Separation and Analysis: Post-adsorption, the adsorbent is separated from the solution. For magnetic composites, this is done simply by applying an external magnet [13]. The remaining concentration of the metal ion in the supernatant is analyzed using techniques like Atomic Absorption Spectroscopy (AAS) or Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES) [97] [13].
- Data Calculation: The adsorption capacity at equilibrium, Qe (mg/g), is calculated as: ( Qe = (Ci - Ce) \times V / m ), where ( Ci ) and ( C_e ) are the initial and equilibrium concentrations (mg/L), respectively, ( V ) is the volume of the solution (L), and ( m ) is the mass of the adsorbent (g).
Synthesis Workflow and Removal Mechanism
The following diagrams illustrate the fundamental processes of creating nanomagnetic chitosan composites and how they function to remove heavy metals.
Figure 1: Two-Step Synthesis Workflow for Magnetic Chitosan Composites. This process, based on co-precipitation and cross-linking, produces a stable and separable adsorbent [13] [47].
Figure 2: Multimodal Heavy Metal Removal Mechanism. Chitosan composites remove metals through several simultaneous interactions, including chelation, ion exchange, and electrostatic attraction [98] [48] [47].
The Scientist's Toolkit: Essential Research Reagents
This table details key materials and their functions for researchers aiming to synthesize and test nanomagnetic chitosan composites based on the protocols cited.
Table 3: Essential Reagents for Synthesis and Adsorption Experiments
| Reagent/Material | Function in Research | Example Source |
|---|---|---|
| Chitosan | Primary biopolymer backbone; provides amino (-NH₂) and hydroxyl (-OH) functional groups for metal binding. | Prawn shells [13], Commercial suppliers [26]. |
| Ferric Chloride (FeCl₃) & Ferrous Sulfate (FeSO₄) | Fe³⁺ and Fe²⁺ precursors for the synthesis of Fe₃O₄ magnetic nanoparticles via co-precipitation. | Sigma-Aldrich [13] [26]. |
| Sodium Hydroxide (NaOH) | Precipitating agent for Fe₃O₄ nanoparticle synthesis; for pH adjustment during adsorption experiments. | Merck [13], Sinopharm [92]. |
| Acetic Acid | Solvent for dissolving chitosan. | Sigma-Aldrich [13]. |
| Sodium Tripolyphosphate (TPP) | Cross-linking agent to enhance the mechanical and chemical stability of chitosan particles. | Sisco Research Laboratories [13]. |
| Heavy Metal Salts (e.g., CdCl₂, Pb(NO₃)₂, K₂Cr₂O₇) | Source of heavy metal ions (Cd²⁺, Pb²⁺, Cr⁶⁺) for preparing synthetic wastewater in adsorption tests. | Sigma-Aldrich [94] [13] [26]. |
| Atomic Absorption Spectroscopy (AAS) | Analytical instrument for quantifying the residual concentration of metal ions in solution after adsorption. | Model GFS97 Thermo Scientific [94]. |
Conclusion
Nanomagnetic chitosan composites represent a cornerstone material for next-generation water purification, successfully merging high adsorption capacity with exceptional operational convenience. Their performance is highly tunable through strategic synthesis and functionalization, enabling targeted removal of specific heavy metals. However, the transition from laboratory success to widespread industrial application requires focused future efforts. Key research directions include standardizing synthesis for batch-to-batch consistency, conducting comprehensive life-cycle assessments and cost analyses, executing long-term pilot-scale studies in real industrial settings, and exploring intelligent composite designs for selective metal recovery and value-added applications.
References
- 1. : Heavy and human health effects | Archives of... metals toxicity [https://link.springer.com/article/10.1007/s00204-024-03903-2]
- 2. A review of toxicity and mechanisms of individual and mixtures of... [https://pubmed.ncbi.nlm.nih.gov/26965280/]
- 3. Current technologies for heavy metal removal from food and environmental resources - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC10786761/]
- 4. Removal of Heavy Metals from Wastewaters: A Challenge from Current Treatment Methods to Nanotechnology Applications - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC7711730/]
- 5. (PDF) Highly efficient of removal from waters by... heavy metals [https://www.academia.edu/122038819/Highly_efficient_removal_of_heavy_metals_from_waters_by_magnetic_chitosan_based_composite]
- 6. Highly efficient of removal from waters by magnetic... heavy metals [https://link.springer.com/article/10.1007/s10450-019-00096-4]
- 7. Chitosan-Coated Magnetic Nanoparticles Prepared in One Step by Reverse Microemulsion Precipitation - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC3821577/]
- 8. Fluorescence Modified Chitosan-Coated Magnetic Nanoparticles for High-Efficient Cellular Imaging - PMC [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2893437/]
- 9. Application Progress of Magnetic Chitosan in Heavy Metal Wastewater Treatment [https://www.mdpi.com/2312-7481/11/9/71]
- 10. as a Biomaterial — Chitosan , Properties, and... | IntechOpen Structure [https://www.intechopen.com/chapters/49246]
- 11. Chitosan-based biosorbents: modification and application for biosorption of heavy metals and radionuclides - PubMed [https://pubmed.ncbi.nlm.nih.gov/24461334/]
- 12. Adsorptive Elimination of Heavy Metals from Aqueous Solution Using Magnetic Chitosan/Cellulose-Fe(III) Composite as a Bio-Sorbent [https://www.mdpi.com/2079-4991/13/10/1595]
- 13. Chitosan-based nano-sorbents: synthesis, surface modification, characterisation and application in Cd (II), Co (II), Cu (II) and Pb (II) ions removal from wastewater | Scientific Reports [https://www.nature.com/articles/s41598-023-32847-3]
- 14. Frontiers | Adsorption of heavy onto food wastes: a review metals [https://www.frontiersin.org/journals/environmental-chemistry/articles/10.3389/fenvc.2025.1526366/full]
- 15. RJPT - Biosorption of Heavy Metals Cu2+, Cd2+, and Pb2+ by Chitosan and Powder from Capiz shell (Placuna placenta) [https://www.rjptonline.org/AbstractView.aspx?PID=2025-18-5-32]
- 16. Enhanced heavy using chemically modified... metals biosorption [https://link.springer.com/article/10.1007/s42452-019-1607-9]
- 17. Heavy Metal Adsorption Using Magnetic Nanoparticles for Water Purification: A Critical Review - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC8707578/]
- 18. Efficient Removal of Heavy Metals, Dyes, and Contaminants from Industrial Wastewater Using Chitosan-Coated Fe3O4 Nanocomposites: Biosynthesis, Characterizations, and Performance Evaluation | Biomass Conversion and Biorefinery [https://link.springer.com/article/10.1007/s13399-024-05526-0]
- 19. Xanthate-Modified Magnetic Fe3O4@SiO2-Based Polyvinyl... [https://pubmed.ncbi.nlm.nih.gov/35335438/]
- 20. Green synthesis and adsorption performance of... [https://pubs.rsc.org/en/content/articlelanding/2025/ra/d5ra00872g]
- 21. Sustainability | Free Full-Text | Preparation of NH2-Functionalized Fe2O3 and Its Chitosan Composites for the Removal of Heavy Metal Ions [https://www.mdpi.com/2071-1050/11/19/5186]
- 22. Green synthesis of Carbonized... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11308582/]
- 23. Separable Fe3O4/ Magnetically - γ 2O3@MIL-88b(Fe) and... | CoLab Fe [https://colab.ws/articles/10.31489%2F2959-0663%2F3-24-5]
- 24. Fe3O4-Polyvinylpyrrolidone-Decorated on Graphene Oxide... [https://jns.kashanu.ac.ir/article_115133.html]
- 25. Selective adsorption of heavy metals from water by a hyper-branched magnetic composite material: Characterization, performance, and mechanism - ScienceDirect [https://www.sciencedirect.com/science/article/abs/pii/S0301479722005527]
- 26. Fabrication of chitosan –magnetite nanocomposite strip for chromium... [https://link.springer.com/article/10.1007/s13204-015-0429-3]
- 27. A novel sodium Iron silicate composite with chitosan for efficient removal of Cd(II) ions from water | Scientific Reports [https://www.nature.com/articles/s41598-025-99232-0]
- 28. Polyvinyl alcohol modified chitosan composite as a novel and efficient adsorbent for multi-metal removal - ScienceDirect [https://www.sciencedirect.com/science/article/abs/pii/S1383586624004702]
- 29. A chitosan -lignin biocomposite adsorbent for RO16 dye and Cr(VI)... [https://bmcchem.biomedcentral.com/articles/10.1186/s13065-025-01621-z]
- 30. -silanol groups functional magnetic nanoparticles for Chitosan ... heavy [https://www.researchsquare.com/article/rs-1177291/v1]
- 31. Fabrication and Characterization of Nanomagnetite/ Chitosan ... [https://link.springer.com/article/10.1007/s10904-024-03204-2]
- 32. Environmental Applications of Chitosan Derivatives and Chitosan Composites | MDPI [https://www.mdpi.com/2073-4360/17/19/2583]
- 33. Modified magnetic chitosan with mono(6-amino-6-deoxy)-β-cyclodextrin as a novel catalyst toward the synthesis of pyrazolopyranopyrimidines and pyrano[2,3-c]pyrazole-3-carboxylates | Scientific Reports [https://www.nature.com/articles/s41598-025-92249-5]
- 34. Advances in nano- chitosan adsorbents and mechanistic insights for... [https://link.springer.com/article/10.1007/s11051-025-06470-4]
- 35. Frontiers | Nano-revolution in heavy metal removal: engineered nanomaterials for cleaner water [https://www.frontiersin.org/journals/environmental-science/articles/10.3389/fenvs.2024.1393694/full]
- 36. Recent research landscape on chitosan-mineral-based composites for wastewater treatment: a comprehensive bibliometric analysis (2014–2024) | Environmental Science and Pollution Research [https://link.springer.com/article/10.1007/s11356-025-36387-3]
- 37. Recent research on landscape -mineral-based chitosan ... composites [https://colab.ws/articles/10.1007%2Fs11356-025-36387-3]
- 38. Enhanced Performance of Chitosan via a Novel Quaternary Magnetic Nanocomposite Chitosan/Grafted Halloysitenanotubes@ZnγFe3O4 for Uptake of Cr (III), Fe (III), and Mn (II) from Wastewater - PubMed [https://pubmed.ncbi.nlm.nih.gov/34451251/]
- 39. Chitosan nanocomposites for the removal of pollutants from waste water | Discover Applied Sciences [https://link.springer.com/article/10.1007/s42452-025-07689-5]
- 40. Hydrogels for Rare Earth Chitosan Extraction Metal [https://discoveryalert.com.au/bio-based-extraction-innovation-2025-sustainable-mining/]
- 41. Removal of heavy metal ions from wastewater: a comprehensive and critical review | npj Clean Water [https://www.nature.com/articles/s41545-021-00127-0]
- 42. Frontiers | Integrated Remediation Processes Toward Heavy Metal Removal/Recovery From Various Environments-A Review [https://www.frontiersin.org/journals/environmental-science/articles/10.3389/fenvs.2019.00066/full]
- 43. Synthesis and characterization of a novel magnetic chitosan–nickel ferrite nanocomposite for antibacterial and antioxidant properties | Scientific Reports [https://www.nature.com/articles/s41598-023-42974-6]
- 44. magnetic chitosan microspheres: Topics by Science.gov [https://www.science.gov/topicpages/m/magnetic+chitosan+microspheres]
- 45. The influence of the polymerization approach on the catalytic... [https://pubs.rsc.org/en/content/articlehtml/2020/ra/d0ra08647a]
- 46. A controllable one-pot hydrothermal of spherical cobalt... synthesis [https://pubs.rsc.org/en/content/articlehtml/2022/ra/d2ra03345c]
- 47. Modified magnetic chitosan materials for heavy metal adsorption: a review - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC9969337/]
- 48. Recent Application Prospects of Chitosan Based Composites for the Metal Contaminated Wastewater Treatment - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC10057141/]
- 49. Application of magnetic chitosan composites for the removal of toxic metal and dyes from aqueous solutions - ScienceDirect [https://www.sciencedirect.com/science/article/abs/pii/S0001868613001176]
- 50. Enhancing mechanical properties of chitosan films via modification with vanillin - PubMed [https://pubmed.ncbi.nlm.nih.gov/26314906/]
- 51. Effect of Selected Crosslinking and Stabilization Methods on the Properties of Porous Chitosan Composites Dedicated for Medical Applications [https://www.mdpi.com/2073-4360/15/11/2507]
- 52. Multifunctional adsorbent based on metal -organic framework... | CoLab [https://colab.ws/articles/10.1016%2Fj.cej.2019.123127]
- 53. Cellulose–Chitosan Functional Biocomposites - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC9863338/]
- 54. Sustainable biomaterials based on cellulose, chitin and chitosan composites - A review - ScienceDirect [https://www.sciencedirect.com/science/article/pii/S2666893921000475]
- 55. Sustainable adsorbents from carboxymethyl... [https://nauchnyimpuls.org/index.php/obrazovaniya/article/view/1324]
- 56. Synthesis of Phosphorus-Modified Magnetic Chitosan and Its Application for Cr(VI) Removal from Aqueous Solution [https://www.mdpi.com/1996-1944/18/21/5019]
- 57. Preparation and adsorption properties of magnetic chitosan/sludge biochar composites for removal of Cu2+ ions | Scientific Reports [https://www.nature.com/articles/s41598-023-46815-4]
- 58. -Al₂O₃ green hydrogel Chitosan : a sustainable approach... composites [https://bmcchem.biomedcentral.com/articles/10.1186/s13065-025-01633-9]
- 59. Synthesis of Magnetic - Chitosan /Fe3O4 Fly for... Ash Composite [https://link.springer.com/article/10.1007/s10924-020-01669-z]
- 60. Adsorption of Heavy Metals: Mechanisms, Kinetics, and Applications of Various Adsorbents in Wastewater Remediation—A Review [https://www.mdpi.com/2813-0391/1/3/46]
- 61. Bio-adsorption of heavy metals from aqueous solution using the ZnO-modified date pits | Scientific Reports [https://www.nature.com/articles/s41598-023-50278-y]
- 62. Comparative Study on the Adsorption Characteristics of Heavy Metal Ions by Activated Carbon and Selected Natural Adsorbents [https://www.mdpi.com/2071-1050/14/23/15579]
- 63. and pseudo Pseudo for malachite... second order kinetics isotherms [https://pubmed.ncbi.nlm.nih.gov/16488537/]
- 64. Removal of heavy metal ions using chitosan and modified chitosan: A review - ScienceDirect [https://www.sciencedirect.com/science/article/abs/pii/S0167732215308801]
- 65. Recent advances in heavy metal removal by chitosan based adsorbents - ScienceDirect [https://www.sciencedirect.com/science/article/abs/pii/S0144861720311735]
- 66. Exploring adsorption dynamics of heavy onto varied... metals [https://pmc.ncbi.nlm.nih.gov/articles/PMC11334899/]
- 67. Enhanced heavy ions metal by 4‑aminobenzoic acid... adsorption [https://scispace.com/papers/enhanced-heavy-metal-ions-adsorption-by-4-aminobenzoic-acid-3pfb5xf7al]
- 68. Enhanced remediation of heavy and dyes from industrial runoff... metals [https://link.springer.com/article/10.1007/s13201-025-02529-8]
- 69. Removal of metal ions using a new magnetic chitosan nano-bio-adsorbent; A powerful approach in water treatment - ScienceDirect [https://www.sciencedirect.com/science/article/abs/pii/S0013935121010471]
- 70. Review of chitosan as a composite adsorbent: heavy ... metal Material [https://pubmed.ncbi.nlm.nih.gov/33673980/]
- 71. of Stability —A Challenge for Pharmaceutical and... Chitosan [https://pmc.ncbi.nlm.nih.gov/articles/PMC4413189/]
- 72. Development of Chitosan Functionalized Magnetic Nanoparticles with Bioactive Compounds - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC7599998/]
- 73. Chitosan-Coated Magnetic Nanoparticles Prepared in One-Step by Precipitation in a High-Aqueous Phase Content Reverse Microemulsion - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC6271106/]
- 74. (PDF) Heavy Ion Adsorbent in Aqueous Solution: A Review on... Metal [https://www.academia.edu/124490080/Heavy_Metal_Ion_Adsorbent_in_Aqueous_Solution_A_Review_on_Chitosan_and_Chitosan_Composites]
- 75. The efficiency of removing heavy metal ions from industrial electropolishing wastewater using natural materials | Scientific Reports [https://www.nature.com/articles/s41598-022-22466-9]
- 76. and Regeneration of non-conventional low-cost... reusability [https://repositorio.unesp.br/entities/publication/a0185bb5-0a66-4d2f-908f-5e128a1f9466]
- 77. Study on Non-functionalized and Thiol-Functionalized... Comparative [https://link.springer.com/article/10.1007/s12668-024-01571-1]
- 78. and Regeneration of non-conventional low-cost... reusability [https://link.springer.com/article/10.1007/s13399-022-03604-9]
- 79. Frontiers | Chitosan and its derivatives-based nanostructures in conjunction with their versatile applications in bio-medicine for alleviating contiguous diseases [https://www.frontiersin.org/journals/materials/articles/10.3389/fmats.2025.1588627/full]
- 80. Statistical optimization of chemical modification of chitosan -magnetic... [https://bnrc.springeropen.com/articles/10.1186/s42269-020-00301-3]
- 81. A Case Study on the Economic Value of Sustainable Design - Clark Nexsen [https://www.clarknexsen.com/blog-case-study-on-the-economic-value-of-sustainable-design/]
- 82. Case studies of sustainable design and eco efficiency: View as single page | OLCreate [https://www.open.edu/openlearncreate/mod/oucontent/view.php?id=5781&printable=1]
- 83. Preparation and adsorption properties of magnetic /sludge... chitosan [https://www.nature.com/articles/s41598-023-46815-4?error=cookies_not_supported&code=584910d1-2045-422d-9ca7-8fe883876147]
- 84. Simulation, prediction and optimization for synthesis and heavy metals adsorption of schwertmannite by machine learning - ScienceDirect [https://www.sciencedirect.com/science/article/abs/pii/S0013935124023788]
- 85. Review: Recent Developments in the Implementation of Activated Carbon as Heavy Metal Removal Management | Water Conservation Science and Engineering [https://link.springer.com/article/10.1007/s41101-024-00287-3]
- 86. Polyaniline@magnetic chitosan nanomaterials for highly efficient simultaneous adsorption and in-situ chemical reduction of hexavalent chromium: Removal efficacy and mechanisms - ScienceDirect [https://www.sciencedirect.com/science/article/abs/pii/S0048969720328333]
- 87. Cysteine-Functionalized Chitosan Magnetic Nano-Based Particles for the Recovery of Light and Heavy Rare Earth Metals: Uptake Kinetics and Sorption Isotherms - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC5312863/]
- 88. Biosorption of heavy metals: Transferability between batch and column studies - ScienceDirect [https://www.sciencedirect.com/science/article/abs/pii/S0045653522001527]
- 89. Superparamagnetic Multifunctionalized Chitosan Nanohybrids for... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10007229/]
- 90. Novel coal fly ash– chitosan for highly composite ... efficient [https://pubs.rsc.org/en/content/articlehtml/2025/ew/d5ew00257e]
- 91. Experimental Design and Breakthrough Curve Modeling of Fixed-Bed Columns Utilizing a Novel 3D Coconut-Based Polyurethane-Activated Carbon Composite Adsorbent for Lead Sequestration [https://www.mdpi.com/2071-1050/15/19/14344]
- 92. Heavy metal removal from aqueous solutions by chitosan-based magnetic composite flocculants - ScienceDirect [https://www.sciencedirect.com/science/article/abs/pii/S1001074221000589]
- 93. Applications of Chitosan in Wastewater | IntechOpen Treatment [https://www.intechopen.com/chapters/52359]
- 94. Lignocellulosic-Based Activated -Loaded Silver Nanoparticles... Carbon [https://pmc.ncbi.nlm.nih.gov/articles/PMC9784523/]
- 95. (PDF) A comparative investigation on removal performances of... [https://www.academia.edu/53153415/A_comparative_investigation_on_removal_performances_of_commercial_activated_carbon_chitosan_biosorbent_and_chitosan_activated_carbon_composite_for_cadmium]
- 96. Application of Activated Banana Peel Coated with... | CoLab Carbon [https://colab.ws/articles/10.3390%2Fma15030860]
- 97. Comparison of heavy metal ions removal from industrial lean amine solvent using ion exchange resins and sand coated with chitosan - ScienceDirect [https://www.sciencedirect.com/science/article/abs/pii/S1875510014000602]
- 98. Recent advances in chitosan -based nanocomposites for adsorption... [https://pubmed.ncbi.nlm.nih.gov/38754671/]